In Vitro Characterization of the Human Skeletal Stem Cell-like Properties of Primary Bone-Derived Mesenchymal Stem/Stromal Cells in Patients with Late and Early Hip Osteoarthritis
- PMID: 35743928
- PMCID: PMC9228448
- DOI: 10.3390/life12060899
In Vitro Characterization of the Human Skeletal Stem Cell-like Properties of Primary Bone-Derived Mesenchymal Stem/Stromal Cells in Patients with Late and Early Hip Osteoarthritis
Abstract
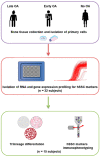
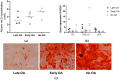
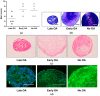
Human skeletal stem cells (hSSCs) were recently identified as podoplanin (PDPN)/CD73/CD164-positive and CD146-negative cells that decline with age, and play a role in the pathogenesis of osteoarthritis (OA). The aim of this study was to identify the hSSC-like properties of bone-derived mesenchymal stem/stromal cells (MSCs) of patients with late and early OA. Methods: First, we performed gene expression profiling for the hSSC markers in 32 patients with late and early OA, and donors without OA. Having identified the low expression of hSSC markers in late OA patients, we further performed trilineage differentiation and immunophenotyping for hSSC makers in the selected subsets from each donor group. Results: Our results show no differences in osteogenesis, chondrogenesis, and adipogenesis between the MSCs from the three groups. However, the immunophenotyping shows lower CD164 in MSCs from early OA patients in comparison with late and no OA subjects (p = 0.002 and p = 0.017). Conclusions: Our study shows that the in vitro hSSC-like properties of bone-derived MSCs are similar in patients with early and late OA, and in donors without OA. However, the lower percentage of CD164-positive MSCs in early OA patients indicates the potential of CD164 as a marker of the onset of OA.
Keywords: CD146; CD164; CD73; bone-derived mesenchymal stem/stromal cells (MSCs); early OA; human skeletal stem cells (hSSCs); immunophenotyping; late osteoarthritis (OA); podoplanin (PDPN); trilineage differentiation.
Conflict of interest statement
The authors declare that they have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Maleitzke T., Elazaly H., Festbaum C., Eder C., Karczewski D., Perka C., Duda G.N., Winkler T. Mesenchymal stromal cell-based therapy—An alternative to arthroplasty for the treatment of osteoarthritis? A state of the art review of clinical trials. J. Clin. Med. 2020;9:2062. doi: 10.3390/jcm9072062. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

