Physical Activity and Post-Transcriptional Regulation of Aging Decay: Modulation of Pathways in Postmenopausal Osteoporosis
- PMID: 35744030
- PMCID: PMC9228623
- DOI: 10.3390/medicina58060767
Physical Activity and Post-Transcriptional Regulation of Aging Decay: Modulation of Pathways in Postmenopausal Osteoporosis
Abstract
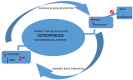
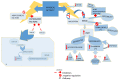
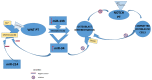
Background and Objectives: Bones and the skeletal muscle play a key role in human physiology as regulators of metabolism in the whole organism. Bone tissue is identified as a complex and dynamic living unit that could react to physical activity. Hormones, growth factors, signaling factors, and environmental factors control osteogenesis, and it could be regulated at a post-transcriptional level. MicroRNAs (miRNAs) can interfere with mRNAs translation. Increasing data suggest that miRNAs, through different pathways, are involved in the regulation of bone marrow mesenchymal stem cells (BMSCs) differentiation and physical activity-induced bone remodeling. The purpose of this narrative review is to investigate the potential protective role played by physical activity in affecting miRNAs expression in close tissues and elaborate on the complex network of interplay that could drive various metabolic responses of the bone to physical activity. Materials and Methods: A bibliographic search of the scientific literature was carried out in scientific databases to investigate the possible effect of physical activity on age-related features detected in the musculoskeletal system. Results: Several studies suggested that the musculoskeletal system interacting at a biomolecular level could establish crosstalk between bone and muscle in an endocrine or paracrine way through myokines released by muscle at the periosteal interface or in the bloodstream, such as irisin. Mechanical stimuli have a key role in bone formation and resorption, increasing osteogenesis and downregulating adipogenesis of BMSC via regulation of expression of runt-related transcription factor 2 (Runx2) and peroxisome proliferator-activated receptor gamma (PPARγ), respectively. Conclusions: Increasing data suggest that miRNAs, through different pathways, are involved in the regulation of BMSCs differentiation and physical activity-induced bone remodeling. Modulation of miRNAs following physical exercise represents an interesting field of investigation since these non-coding RNAs may be considered defenders against degenerative diseases and as well as useful prognostic markers in skeletal and muscle-skeletal diseases, such as osteoporosis.
Keywords: continuous moderate-intensity exercise; high-intensity interval exercise; irisin; miRNAs; osteoporosis; physical activity; resistance exercise; training.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Yen K.L., Capilla E., Rosen C.J., Gilsanz V., Pessin J.E., Judex S., Rubin C.T. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J. Bone Miner. Res. 2009;24:50–61. doi: 10.1359/jbmr.080817. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

