Clinical Management of Acromegaly: Therapeutic Frontiers and New Perspectives for Somatostatin Receptor Ligands (SRLs)
- PMID: 35744057
- PMCID: PMC9228014
- DOI: 10.3390/medicina58060794
Clinical Management of Acromegaly: Therapeutic Frontiers and New Perspectives for Somatostatin Receptor Ligands (SRLs)
Abstract
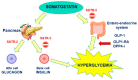
Somatostatin receptor ligands (SRLs) represent a true milestone in the medical therapy for acromegaly. The first-generation SRLs (FG-SRLs), octreotide and lanreotide, have demonstrated good efficacy in disease control and tumor shrinkage, and are still considered first-line medical therapies. The development of long-acting release (LAR) formulations has certainly improved the therapeutic tolerability of these drugs, although many patients still experience therapy-related burden. As such, new formulations have recently been developed to improve adherence and therapeutic efficacy and more solutions are on the way. In the case of FG-SRL-resistant disease, pasireotide, the only second generation SRL currently available, demonstrated superiority in disease control and tumor shrinkage compared to FG-SRLs. However, its use in clinical practice is still limited due to concern for impairment in glucose homeostasis. In this review, we discuss the news about the present and future role of SRLs in acromegaly, exploring the therapeutical frontiers of this drug class. Moreover, we provide practical guidance on the use of pasireotide, based on the data in the literature and our clinical experience.
Keywords: SSA; acromegaly; pituitary; somatostatin; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Future of Somatostatin Receptor Ligands in Acromegaly.J Clin Endocrinol Metab. 2022 Jan 18;107(2):297-308. doi: 10.1210/clinem/dgab726. J Clin Endocrinol Metab. 2022. PMID: 34618894 Free PMC article. Review.
-
Pasireotide: a novel treatment for patients with acromegaly.Drug Des Devel Ther. 2016 Jan 11;10:227-39. doi: 10.2147/DDDT.S77999. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 26811671 Free PMC article. Review.
-
Pasireotide: potential treatment option for McCune-Albright-associated acromegaly.Eur J Endocrinol. 2024 Jan 3;190(1):K17-K20. doi: 10.1093/ejendo/lvad173. Eur J Endocrinol. 2024. PMID: 38128124
-
The Effect of 6 Months' Treatment With Pasireotide LAR on Glucose Metabolism in Patients With Resistant Acromegaly in Real-World Clinical Settings.Front Endocrinol (Lausanne). 2021 Mar 10;12:633944. doi: 10.3389/fendo.2021.633944. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33776927 Free PMC article.
-
Pasireotide in the Personalized Treatment of Acromegaly.Front Endocrinol (Lausanne). 2021 Mar 16;12:648411. doi: 10.3389/fendo.2021.648411. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33796079 Free PMC article. Review.
Cited by
-
Immunohistochemical evaluation of biomarkers with predictive role in acromegaly: a literature review.Rom J Morphol Embryol. 2023 Jan-Mar;64(1):25-33. doi: 10.47162/RJME.64.1.03. Rom J Morphol Embryol. 2023. PMID: 37128788 Free PMC article. Review.
-
Approach of Acromegaly during Pregnancy.Diagnostics (Basel). 2022 Nov 2;12(11):2669. doi: 10.3390/diagnostics12112669. Diagnostics (Basel). 2022. PMID: 36359512 Free PMC article. Review.
References
-
- Giustina A., Barkhoudarian G., Beckers A., Ben-Shlomo A., Biermasz N., Biller B., Boguszewski C., Bolanowski M., Bollerslev J., Bonert V., et al. Multidisciplinary management of acromegaly: A consensus. Rev. Endocr. Metab. Disord. 2020;21:667–678. doi: 10.1007/s11154-020-09588-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous