A Critical Review on the Sensing, Control, and Manipulation of Single Molecules on Optofluidic Devices
- PMID: 35744582
- PMCID: PMC9229244
- DOI: 10.3390/mi13060968
A Critical Review on the Sensing, Control, and Manipulation of Single Molecules on Optofluidic Devices
Abstract
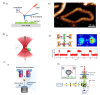
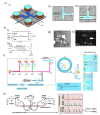
Single-molecule techniques have shifted the paradigm of biological measurements from ensemble measurements to probing individual molecules and propelled a rapid revolution in related fields. Compared to ensemble measurements of biomolecules, single-molecule techniques provide a breadth of information with a high spatial and temporal resolution at the molecular level. Usually, optical and electrical methods are two commonly employed methods for probing single molecules, and some platforms even offer the integration of these two methods such as optofluidics. The recent spark in technological advancement and the tremendous leap in fabrication techniques, microfluidics, and integrated optofluidics are paving the way toward low cost, chip-scale, portable, and point-of-care diagnostic and single-molecule analysis tools. This review provides the fundamentals and overview of commonly employed single-molecule methods including optical methods, electrical methods, force-based methods, combinatorial integrated methods, etc. In most single-molecule experiments, the ability to manipulate and exercise precise control over individual molecules plays a vital role, which sometimes defines the capabilities and limits of the operation. This review discusses different manipulation techniques including sorting and trapping individual particles. An insight into the control of single molecules is provided that mainly discusses the recent development of electrical control over single molecules. Overall, this review is designed to provide the fundamentals and recent advancements in different single-molecule techniques and their applications, with a special focus on the detection, manipulation, and control of single molecules on chip-scale devices.
Keywords: fluorescence; lab-on-a-chip; microfluidics; nanopore; optofluidics; single-molecule method.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

