Effect of Caging on Cryptosporidium parvum Proliferation in Mice
- PMID: 35744762
- PMCID: PMC9230662
- DOI: 10.3390/microorganisms10061242
Effect of Caging on Cryptosporidium parvum Proliferation in Mice
Abstract
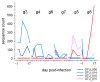
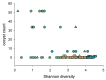
Cryptosporidiosis is an enteric infection caused by several protozoan species in the genus Cryptosporidium (phylum Apicomplexa). Immunosuppressed mice are commonly used to model this infection. Surprisingly, for a pathogen like Cryptosporidium parvum, which is readily transmitted fecal-orally, mice housed in the same cage can develop vastly different levels of infection, ranging from undetectable to lethal. The motivation for this study was to investigate this phenomenon and assess the association between the severity of cryptosporidiosis and the fecal microbiota. To this aim, the association between severity of cryptosporidiosis and caging (group caged vs. individually caged) and between the microbiota taxonomy and the course of the infection was examined. In contrast to mice caged in groups of four, a majority of mice caged individually did not excrete a detectable level of oocysts. Microbiota α diversity in samples collected between three days prior to infection and one day post-infection was negatively correlated with the severity of cryptosporidiosis, suggesting a causal negative relationship between microbiota diversity and susceptibility to C. parvum.
Keywords: Cryptosporidium; constrained ordination; cryptosporidiosis; dysbiosis; microbiota.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Kotloff K.L., Blackwelder W.C., Nasrin D., Nataro J.P., Farag T.H., van Eijk A., Adegbola R.A., Alonso P.L., Breiman R.F., Faruque A.S., et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: Epidemiologic and clinical methods of the case/control study. Clin. Infect. Dis. 2012;55((Suppl. 4)):S232–S245. doi: 10.1093/cid/cis753. - DOI - PMC - PubMed
-
- Checkley W., White A.C., Jr., Jaganath D., Arrowood M.J., Chalmers R.M., Chen X.M., Fayer R., Griffiths J.K., Guerrant R.L., Hedstrom L., et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect. Dis. 2015;15:85–94. doi: 10.1016/S1473-3099(14)70772-8. - DOI - PMC - PubMed
-
- Shikani H., Weiss L.M. Cryptosporidium: Parasite and Disease. Springer; Berlin/Heidelberg, Germany: 2014. Human cryptosporidiosis: A clinical perspective; pp. 383–421.
Grants and funding
LinkOut - more resources
Full Text Sources

