The Ameliorative Effect of Empagliflozin in Vigabatrin-Induced Cerebellar/Neurobehavioral Deficits: Targeting mTOR/AMPK/SIRT-1 Signaling Pathways
- PMID: 35744783
- PMCID: PMC9229258
- DOI: 10.3390/molecules27123659
The Ameliorative Effect of Empagliflozin in Vigabatrin-Induced Cerebellar/Neurobehavioral Deficits: Targeting mTOR/AMPK/SIRT-1 Signaling Pathways
Abstract
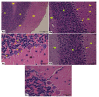
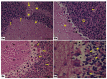
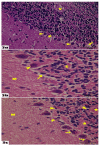
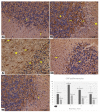
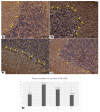
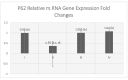
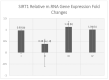
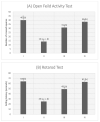
Introduction. Vigabatrin (VGB) is an antiepileptic drug that acts to irreversibly inhibit the γ-aminobutyric acid (GABA) transaminase enzyme, elevating GABA levels. Broad studies have established that long-term treatment and/or high doses of VGB lead to variable visual defects. However, little attention has been paid to its other side effects, especially those demonstrating cerebellar involvement. Sodium glucose-linked co-transporter 2 (SGLT2) inhibitors are antidiabetic agents with protective effects far greater than expected based on their anti-hyperglycemic effect. Method. Our study herein was designed to investigate the possible ameliorative effect of empagliflozin, the SGLT2 inhibitors, in VGB-induced cerebellar toxicity. A total of 40 male Wistar rats were allocated equally into 4 groups: Group I: control group; Group II: VGB group; Group III empagliflozin treated VGB group; and Group IV: empagliflozin treated group. All groups were subjected to the detection of cerebellar messenger RNA gene expression of silent mating type information regulation 2 homolog 1 (SIRT1) and Nucleoporin p62 (P62). Mammalian target of rapamycin (mTOR), adenosine monophosphate-activated protein kinase (AMPK), and beclin1 levels were assessed by the ELISA technique while malondialdehyde (MDA) level and superoxide dismutase (SOD) activity were detected spectrophotometrically. Immuno-histochemical studies, focusing on glial fibrillary acidic protein (GFAP) and S100 were performed, and the optical color density and the mean area percentage of GFAP positive astrocytes and the number of S 100 positive cells were also counted. Results. Following empagliflozin treatment, we documented significant upregulation of both SIRT1 and P62 mRNA gene expression. Additionally, AMPK, Beclin1 levels, and SOD activity were significantly improved, while both mTOR and MDA levels were significantly reduced. Conclusions. We concluded for the first time that empagliflozin efficiently ameliorated the VGB-induced disrupted mTOR/AMPK/SIRT-1 signaling axis with subsequent improvement of the autophagy machinery and mitigation of the oxidative and inflammatory cellular environment, paving the way for an innovative therapeutic potential in managing VGB-induced neurotoxicity.
Keywords: AMPK; GFAP; SIRT-1; empagliflozin; mTOR; vigabatrin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Singh D., Jethani S., Dubey A., Deepa S., Aksh D. Vigabatrin induced intramyelinic oedema in cerebellum of albino rats. J. Anat. Soc. India. 2014;63:156–160. doi: 10.1016/j.jasi.2014.11.008. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

