Tripeptide IRW Protects MC3T3-E1 Cells against Ang II Stress in an AT2R Dependent Manner
- PMID: 35744810
- PMCID: PMC9230126
- DOI: 10.3390/molecules27123684
Tripeptide IRW Protects MC3T3-E1 Cells against Ang II Stress in an AT2R Dependent Manner
Abstract
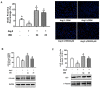
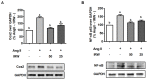
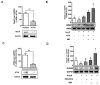
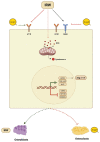
Multiple strategies including the use of bioactive peptides and other nutraceuticals are being adopted to maintain bone health. This study provides an improved and deeper understanding of the pharmacological effects that a bioactive peptide IRW (Ile-Arg-Trp) extends on bone health. Our results showed that IRW treatment protects osteoblasts against Ang II induced decline in cell proliferation and restores protein levels of collagen type I alpha 2 chain (COL1A2) and alkaline phosphatase (ALP) levels in MC3T3-E1 cells (p < 0.05). Apart from augmentation of these mineralization factors, the angiotensin II (Ang II) induced apoptotic stress in osteoblasts was mitigated by IRW as well. At the molecular level, IRW abolished the cytochrome-c release via modulation of pro-and anti-apoptotic genes in MC3T3-E1 cells (p < 0.05). Interestingly, IRW also increased cellular levels of cytoprotective local RAAS factors such as MasR, Ang (1−7), ACE2, and AT2R, and lowered the levels of Ang II effector receptor (AT1R). Further, our results indicated a lower content of inflammation and osteoclastogenesis biomarkers such as cyclooxygenase 2 (COX2), nuclear factor kappa B (NF-κB), and receptor activator of nuclear factor kappa-B ligand (RANKL) following IRW treatment in MC3T3-E1 cells (p < 0.05). The use of an antagonist-guided cell study indicated that IRW contributed to the process of cytoprotection and proliferation of osteoblasts via Runt-related transcription factor 2 (RUNX2) in face of Ang II stress in an AT2R dependent manner. The key findings of our study showed that IRW could potentially have a therapeutic role in the treatment and/or prevention of bone disorders.
Keywords: AT2R; IRW; RAAS; RUNX2; osteoporosis; peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Tripeptide IRW initiates differentiation in osteoblasts differentiation via the RUNX2 pathway.Biochim Biophys Acta Gen Subj. 2019 Jun;1863(6):1138-1146. doi: 10.1016/j.bbagen.2019.04.007. Epub 2019 Apr 10. Biochim Biophys Acta Gen Subj. 2019. PMID: 30980895
-
Tripeptide IRW (Ile-Arg-Trp) as a Potential Nutraceutical Intervention in Osteoporosis.J Nutr Sci Vitaminol (Tokyo). 2022;68(Supplement):S113-S115. doi: 10.3177/jnsv.68.S113. J Nutr Sci Vitaminol (Tokyo). 2022. PMID: 36436988
-
Modulatory Effects of Egg White Ovotransferrin-Derived Tripeptide IRW (Ile-Arg-Trp) on Vascular Smooth Muscle Cells against Angiotensin II Stimulation.J Agric Food Chem. 2016 Oct 5;64(39):7342-7347. doi: 10.1021/acs.jafc.6b03513. Epub 2016 Sep 27. J Agric Food Chem. 2016. PMID: 27649793
-
A Novel Angiotensin Converting Enzyme 2 (ACE2) Activating Peptide: A Reflection of 10 Years of Research on a Small Peptide Ile-Arg-Trp (IRW).J Agric Food Chem. 2020 Dec 9;68(49):14402-14408. doi: 10.1021/acs.jafc.0c05544. Epub 2020 Nov 29. J Agric Food Chem. 2020. PMID: 33251800 Review.
-
The ACE2/Ang (1-7)/MasR axis as an emerging target for antihypertensive peptides.Crit Rev Food Sci Nutr. 2021;61(15):2572-2586. doi: 10.1080/10408398.2020.1781049. Epub 2020 Jun 18. Crit Rev Food Sci Nutr. 2021. PMID: 32551837 Review.
References
-
- Morgan E.F., Gerstenfeld L.C. Marcus and Feldman’s Osteoporosis. Elsevier; Amsterdam, The Netherlands: 2021. The bone organ system: Form and function; pp. 15–35.
-
- Bislev L.S., Sikjær T., Rolighed L., Rejnmark L. Relationship Between Aldosterone and Parathyroid Hormone, and the Effect of Angiotensin and Aldosterone Inhibition on Bone Health. Clin. Rev. Bone Miner. Metab. 2015;13:194–205. doi: 10.1007/s12018-015-9182-0. - DOI
-
- Fountain J.H., Lappin S.L. Physiology, Renin Angiotensin System. StatPearls Publishing; Treasure Island, FL, USA: 2017. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

