Combined Second Harmonic Generation and Fluorescence Analyses of the Structures and Dynamics of Molecules on Lipids Using Dual-Probes: A Review
- PMID: 35744902
- PMCID: PMC9231091
- DOI: 10.3390/molecules27123778
Combined Second Harmonic Generation and Fluorescence Analyses of the Structures and Dynamics of Molecules on Lipids Using Dual-Probes: A Review
Abstract
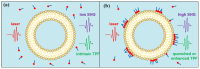
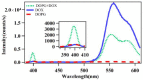
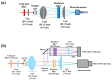
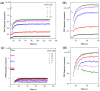
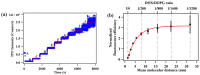
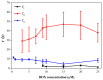
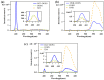
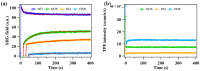
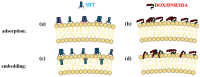
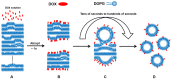
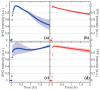
Revealing the structures and dynamic behaviors of molecules on lipids is crucial for understanding the mechanism behind the biophysical processes, such as the preparation and application of drug delivery vesicles. Second harmonic generation (SHG) has been developed as a powerful tool to investigate the molecules on various lipid membranes, benefiting from its natural property of interface selectivity, which comes from the principle of even order nonlinear optics. Fluorescence emission, which is in principle not interface selective but varies with the chemical environment where the chromophores locate, can reveal the dynamics of molecules on lipids. In this contribution, we review some examples, which are mainly from our recent works focusing on the application of combined spectroscopic methods, i.e., SHG and two-photon fluorescence (TPF), in studying the dynamic behaviors of several dyes or drugs on lipids and surfactants. This review demonstrates that molecules with both SHG and TPF efficiencies may be used as intrinsic dual-probes in plotting a clear physical picture of their own behaviors, as well as the dynamics of other molecules, on lipid membranes.
Keywords: dual-probes; lipid membrane; second harmonic generation; two-photon fluorescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
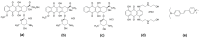





Similar articles
-
Differences in Lipid Order and Dynamics in Plasma Membranes Assessed by Nonlinear Optical Microscopy.J Phys Chem B. 2024 Feb 22;128(7):1680-1688. doi: 10.1021/acs.jpcb.3c06725. Epub 2024 Feb 12. J Phys Chem B. 2024. PMID: 38347710
-
Monitoring the response of a model protocell to dye and surfactant molecules through second harmonic generation and fluorescence imaging.Phys Chem Chem Phys. 2024 Mar 6;26(10):8148-8157. doi: 10.1039/d4cp00009a. Phys Chem Chem Phys. 2024. PMID: 38380536
-
Unveiling the Molecular Dynamics in a Living Cell to the Subcellular Organelle Level Using Second-Harmonic Generation Spectroscopy and Microscopy.Anal Chem. 2021 Oct 26;93(42):14146-14152. doi: 10.1021/acs.analchem.1c02604. Epub 2021 Oct 14. Anal Chem. 2021. PMID: 34648265
-
Optical second harmonic generation microscopy: application to the sensitive detection of cell membrane damage.Biophys Rev. 2019 Jun;11(3):399-408. doi: 10.1007/s12551-019-00546-x. Epub 2019 May 9. Biophys Rev. 2019. PMID: 31073956 Free PMC article. Review.
-
Applications of second harmonic generation (SHG)/sum-frequency generation (SFG) imaging for biophysical characterization of the plasma membrane.Biophys Rev. 2020 Oct 27;12(6):1321-9. doi: 10.1007/s12551-020-00768-4. Online ahead of print. Biophys Rev. 2020. PMID: 33108561 Free PMC article. Review.
Cited by
-
Boosting Second Harmonic Generation Efficiency and Nonlinear Susceptibility via Metasurfaces Featuring Split-Ring Resonators and Bowtie Nanoantennas.Nanomaterials (Basel). 2024 Apr 11;14(8):664. doi: 10.3390/nano14080664. Nanomaterials (Basel). 2024. PMID: 38668158 Free PMC article.
-
The Micro-Structure of the Celiac Ganglia-A Two-Photon Microscopy Study on Parkinson's Disease.Diagnostics (Basel). 2025 Mar 8;15(6):659. doi: 10.3390/diagnostics15060659. Diagnostics (Basel). 2025. PMID: 40150001 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

