Observation of Hyperpositive Non-Linear Effect in Asymmetric Organozinc Alkylation in Presence of N-Pyrrolidinyl Norephedrine
- PMID: 35744906
- PMCID: PMC9230045
- DOI: 10.3390/molecules27123780
Observation of Hyperpositive Non-Linear Effect in Asymmetric Organozinc Alkylation in Presence of N-Pyrrolidinyl Norephedrine
Erratum in
-
Correction: Thierry et al. Observation of Hyperpositive Non-Linear Effect in Asymmetric Organozinc Alkylation in Presence of N-Pyrrolidinyl Norephedrine. Molecules 2022, 27, 3780.Molecules. 2024 Sep 9;29(17):4265. doi: 10.3390/molecules29174265. Molecules. 2024. PMID: 39275129 Free PMC article.
Abstract
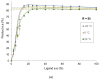
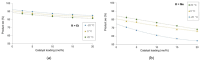
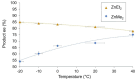
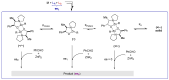
Phenomena related to asymmetric amplification are considered to be key to understanding the emergence of homochirality in life. In asymmetric catalysis, theoretical and experimental models have been studied to understand such chiral amplification, in particular based on non-linear effects. Three decades after the theoretical demonstration that a chiral catalyst, when not enantiopure, could be more enantioselective than its enantiopure counterpart, we show here a new experimental example of nonlinear hyperpositive effect. We report here our investigations in the enantioselective zinc-catalyzed alkylation of benzaldehyde with N-pyrrolidinyl norephedrine as partially resolved chiral ligand, which shows a significant hyperpositive non-linear effect. A study of the underlying mechanism was conducted, which allows us to confirm a mechanism that implies a monomeric and a dimeric complex both catalyzing the reaction at a steady state and giving different enantioselectivities.
Keywords: asymmetric catalysis; chiral amplification; nonlinear effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

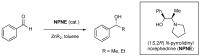



References
-
- Jacobsen E.N., Pfaltz A., Yamamoto H. Comprehensive Asymmetric Catalysis. Springer; Berlin/Heidelberg, Germany: 1999.
-
- Guillaneux D., Zhao S.-H., Samuel O., Rainford D., Kagan H.B. Nonlinear effects in asymmetric catalysis. J. Am. Chem. Soc. 1994;116:9430–9439. doi: 10.1021/ja00100a004. - DOI
-
- Geiger Y., Achard T., Maisse-François A., Bellemin-Laponnaz S. Hyperpositive nonlinear effects in asymmetric catalysis. Nat. Catal. 2020;3:422–426. doi: 10.1038/s41929-020-0441-1. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

