Imidazo[1,5- a]pyridine-Based Fluorescent Probes: A Photophysical Investigation in Liposome Models
- PMID: 35744979
- PMCID: PMC9230927
- DOI: 10.3390/molecules27123856
Imidazo[1,5- a]pyridine-Based Fluorescent Probes: A Photophysical Investigation in Liposome Models
Abstract
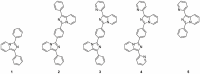
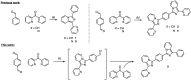
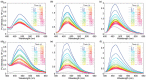
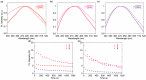
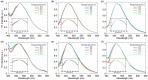
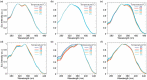
Imidazo[1,5-a]pyridine is a stable scaffold, widely used for the development of emissive compounds in many application fields (e.g., optoelectronics, coordination chemistry, sensors, chemical biology). Their compact shape along with remarkable photophysical properties make them suitable candidates as cell membrane probes. The study of the membrane dynamics, hydration, and fluidity is of importance to monitor the cellular health and to explore crucial biochemical pathways. In this context, five imidazo[1,5-a]pyridine-based fluorophores were synthesized according to a one-pot cyclization between an aromatic ketone and benzaldehyde in the presence of ammonium acetate and acetic acid. The photophysical features of prepared compounds were investigated in several organic solvents and probes 2-4 exhibited the greatest solvatochromic behavior, resulting in a higher suitability as membrane probes. Their interaction with liposomes as artificial membrane model was tested showing a successful intercalation of the probes in the lipid bilayer. Kinetic experiments were carried out and the lipidic phase influence on the photophysical features was evaluated through temperature-dependent experiments. The results herein reported encourage further investigations on the use of imidazo[1,5-a]pyridine scaffold as fluorescent membrane probes.
Keywords: fluorescence; imidazo[1,5-a]pyridine; large Stokes shift; liposome; membrane probes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Volpi G., Rabezzana R. Imidazo[1,5-a]pyridine derivatives: Useful, luminescent and versatile scaffolds for different applications. New J. Chem. 2021;45:5737–5743. doi: 10.1039/D1NJ00322D. - DOI
-
- Colombo G., Ardizzoia G.A., Brenna S. Imidazo[1,5-a]pyridine-based derivatives as highly fluorescent dyes. Inorg. Chim. Acta. 2022;535:120849. doi: 10.1016/j.ica.2022.120849. - DOI
-
- Fresta E., Volpi G., Garino C., Barolo C., Costa R.D. Contextualizing yellow light-emitting electrochemical cells based on a blue-emitting imidazo-pyridine emitter. Polyhedron. 2018;140:129–137. doi: 10.1016/j.poly.2017.11.048. - DOI
-
- Volpi G. Luminescent imidazo[1,5-a]pyridine scaffold: Synthetic heterocyclization strategies overview and promising applications. Asian J. Org. Chem. 2022 doi: 10.1002/ajoc.202200171. - DOI

