Discovery of Novel Quinazoline Derivatives as Potent Antitumor Agents
- PMID: 35745027
- PMCID: PMC9230651
- DOI: 10.3390/molecules27123906
Discovery of Novel Quinazoline Derivatives as Potent Antitumor Agents
Abstract
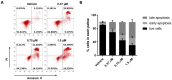
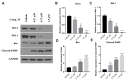
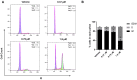
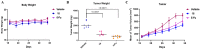
In this work, we designed and synthesized a novel series of quinazoline derivatives 6-19 and then evaluated their broad-spectrum antitumor activity against MGC-803, MCF-7, PC-9, A549, and H1975, respectively. Most of them demonstrated low micromolar cytotoxicity towards five tested cell lines. In particular, compound 18 exhibited nanomolar level inhibitory activity against MGC-803 cells with an IC50 value of 0.85 μM, indicating approximately a 32-fold selectivity against GES-1 (IC50 = 26.75 μM). Further preclinical evaluation showed that compound 18 remarkably inhibited the migration of MGC-803 cells, induced cell cycle arrest at G2/M, and induced MGC-803 apoptosis, resulting in decreasing the expression of both Bcl-2 and Mcl-1, and up-regulating the expression of both Bax and cleaved PARP. No death or obvious pathological damage was observed in mice by acute toxicity assay. The in vivo antitumor evaluation suggested that compound 18 significantly decreased the average tumor volume and tumor weight without any effect on body weight, which is better than 5-Fu. Therefore, compound 18 can be used as a lead compound for the further development of antitumor drugs in the future.
Keywords: antitumor; apoptosis; cell cycle; migration; quinazoline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Design, synthesis and biological evaluation of novel 2,4-disubstituted quinazoline derivatives targeting H1975 cells via EGFR-PI3K signaling pathway.Bioorg Med Chem. 2021 Aug 1;43:116265. doi: 10.1016/j.bmc.2021.116265. Epub 2021 Jun 27. Bioorg Med Chem. 2021. PMID: 34192644
-
Design, Synthesis, and Biological Evaluation of Novel Quinazoline Derivatives Possessing a Trifluoromethyl Moiety as Potential Antitumor Agents.Chem Biodivers. 2024 May;21(5):e202301776. doi: 10.1002/cbdv.202301776. Epub 2024 Apr 11. Chem Biodivers. 2024. PMID: 38602834
-
Development of a series of novel 4-anlinoquinazoline derivatives possessing quinazoline skeleton: Design, synthesis, EGFR kinase inhibitory efficacy, and evaluation of anticancer activities in vitro.Eur J Med Chem. 2017 Sep 29;138:669-688. doi: 10.1016/j.ejmech.2017.07.005. Epub 2017 Jul 4. Eur J Med Chem. 2017. PMID: 28711702
-
2,4-Disubstituted quinazolines targeting breast cancer cells via EGFR-PI3K.Eur J Med Chem. 2019 Jun 15;172:36-47. doi: 10.1016/j.ejmech.2019.03.030. Epub 2019 Mar 17. Eur J Med Chem. 2019. PMID: 30939352
-
Synthesis and Biological Evaluation of Steviol Derivatives with Improved Cytotoxic Activity and Selectivity.J Nat Prod. 2022 Aug 26;85(8):1945-1958. doi: 10.1021/acs.jnatprod.2c00161. Epub 2022 Aug 9. J Nat Prod. 2022. PMID: 35943432 Review.
Cited by
-
6-Bromo quinazoline derivatives as cytotoxic agents: design, synthesis, molecular docking and MD simulation.BMC Chem. 2024 Jul 4;18(1):125. doi: 10.1186/s13065-024-01230-2. BMC Chem. 2024. PMID: 38965630 Free PMC article.
-
New 6-nitro-4-substituted quinazoline derivatives targeting epidermal growth factor receptor: design, synthesis and in vitro anticancer studies.Future Med Chem. 2024;16(19):2025-2041. doi: 10.1080/17568919.2024.2389772. Epub 2024 Sep 4. Future Med Chem. 2024. PMID: 39230501 Free PMC article.
References
-
- Mubeen M., Kini S.G. A Review on: The Design and Development of EGFR Tyrosine Kinase Inhibitors in Cancer Therapy. Int. J. Ther. Appl. 2012;5:29–37.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

