Dipeptidyl Peptidase 3 Activity as a Promising Biomarker of Bone Fragility in Postmenopausal Women
- PMID: 35745051
- PMCID: PMC9227407
- DOI: 10.3390/molecules27123929
Dipeptidyl Peptidase 3 Activity as a Promising Biomarker of Bone Fragility in Postmenopausal Women
Abstract
The dipeptidyl peptidase 3 (Dpp3) is a ubiquitous zinc-dependent aminopeptidase, participating in the activation or degradation of signaling peptides and in the Keap1−Nrf2 antioxidant pathway. The absence of Dpp3 in the Dpp3 knockout mouse model causes increased osteoclast activity, altered osteogenic function, sustained oxidative stress in the bone tissue, and bone loss. We aimed to assess the association of Dpp3 activity with bone fragility in postmenopausal osteoporosis and the impact of denosumab on enzymatic activity. We conducted a two-phase study including 69 postmenopausal women with severe osteoporosis and 36 postmenopausal women without osteometabolic conditions, as controls (cross-sectional phase). Subjects with severe osteoporosis were assessed at baseline and 14 days after the first denosumab administration (prospective phase). The results showed significant reduction in serum Dpp3 activity (expressed as nmoles of formed product/mg proteins/min) in patients vs. controls (0.791 ± 0.232 vs. 1.195 ± 0.338; p < 0.001), and significant association with bone mass at the femoral neck (r = 0.28, p = 0.02) in patients prior to treatment. We found a negative correlation between C-terminal telopeptide (CTX) or N-terminal pro-peptide of type 1 procollagen (P1NP) levels and Dpp3 activity (respectively, r = −0.29, p = 0.012; and r = −0.2572, p = 0.033). Dpp3 activity did not change after denosumab injection. Our findings support a critical role played by Dpp3 in bone homeostasis as a potential bone protective factor. Additional clinical studies in larger cohorts might explore the implementation of Dpp3 assessment as a biomarker of bone health status.
Keywords: Dpp3; bone mineral density; fragility fracture; osteoporosis; oxidative stress; serum biomarker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

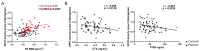


Similar articles
-
Absence of Dipeptidyl Peptidase 3 Increases Oxidative Stress and Causes Bone Loss.J Bone Miner Res. 2019 Nov;34(11):2133-2148. doi: 10.1002/jbmr.3829. Epub 2019 Sep 9. J Bone Miner Res. 2019. PMID: 31295380 Free PMC article.
-
Dipeptidyl peptidase 3 is essential for maintaining osteoblastic differentiation under a high-glucose environment by inhibiting apoptosis, oxidative stress and inflammation through the modulation of the Keap1-Nrf2 pathway.Int Immunopharmacol. 2023 Jul;120:110404. doi: 10.1016/j.intimp.2023.110404. Epub 2023 Jun 3. Int Immunopharmacol. 2023. PMID: 37276831
-
Dipeptidyl-peptidase 3 protects oxygen-glucose deprivation/reoxygenation-injured hippocampal neurons by suppressing apoptosis, oxidative stress and inflammation via modulation of Keap1/Nrf2 signaling.Int Immunopharmacol. 2021 Jul;96:107595. doi: 10.1016/j.intimp.2021.107595. Epub 2021 Mar 31. Int Immunopharmacol. 2021. PMID: 33812256
-
The emerging role of dipeptidyl peptidase 3 in pathophysiology.FEBS J. 2023 May;290(9):2246-2262. doi: 10.1111/febs.16429. Epub 2022 Mar 25. FEBS J. 2023. PMID: 35278345 Review.
-
Efficacy and Safety of Denosumab in Postmenopausal Women With Osteoporosis: A Meta-Analysis.Medicine (Baltimore). 2015 Nov;94(44):e1674. doi: 10.1097/MD.0000000000001674. Medicine (Baltimore). 2015. PMID: 26554766 Free PMC article. Review.
Cited by
-
Proteomic Biomarkers Associated with Low Bone Mineral Density: A Systematic Review.Int J Mol Sci. 2024 Jul 9;25(14):7526. doi: 10.3390/ijms25147526. Int J Mol Sci. 2024. PMID: 39062769 Free PMC article.
-
DPP3: From biomarker to therapeutic target of cardiovascular diseases.Front Cardiovasc Med. 2022 Oct 12;9:974035. doi: 10.3389/fcvm.2022.974035. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36312232 Free PMC article. Review.
-
Label-free multimodal nonlinear optical microscopy reveals features of bone composition in pathophysiological conditions.Front Bioeng Biotechnol. 2022 Nov 22;10:1042680. doi: 10.3389/fbioe.2022.1042680. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36483771 Free PMC article.
References
-
- Lu K., Alcivar A.L., Ma J., Foo T.K., Zywea S., Mahdi A., Huo Y., Kensler T.W., Gatza M.L., Xia B. NRF2 Induction Supporting Breast Cancer Cell Survival Is Enabled by Oxidative Stress-Induced Dpp3-KEAP1 Interaction. Cancer Res. 2017;77:2881–2892. doi: 10.1158/0008-5472.CAN-16-2204. - DOI - PMC - PubMed
-
- Ren X., Yu J., Guo L., Ma H. Dipeptidyl-Peptidase 3 Protects Oxygen-Glucose Deprivation/Reoxygenation-Injured Hippocampal Neurons by Suppressing Apoptosis, Oxidative Stress and Inflammation via Modulation of Keap1/Nrf2 Signaling. Int. Immunopharmacol. 2021;96:107595. doi: 10.1016/j.intimp.2021.107595. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

