Synthesis and Antileukemia Activity Evaluation of Benzophenanthridine Alkaloid Derivatives
- PMID: 35745057
- PMCID: PMC9227418
- DOI: 10.3390/molecules27123934
Synthesis and Antileukemia Activity Evaluation of Benzophenanthridine Alkaloid Derivatives
Abstract
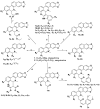
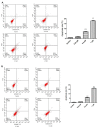
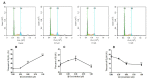
Thirty-three benzophenanthridine alkaloid derivatives (1a-1u and 2a-2l) were synthesized, and their cytotoxic activities against two leukemia cell lines (Jurkat Clone E6-1 and THP-1) were evaluated in vitro using a Cell Counting Kit-8 (CCK-8) assay. Nine of these derivatives (1i-l, 2a, and 2i-l) with IC50 values in the range of 0.18-7.94 μM showed significant inhibitory effects on the proliferation of both cancer cell lines. Analysis of the primary structure-activity relationships revealed that different substituent groups at the C-6 position might have an effect on the antileukemia activity of the corresponding compounds. In addition, the groups at the C-7 and C-8 positions could influence the antileukemia activity. Among these compounds, 2j showed the strongest in vitro antiproliferative activity against Jurkat Clone E6-1 and THP-1 cells with good IC50 values (0.52 ± 0.03 μM and 0.48 ± 0.03 μM, respectively), slightly induced apoptosis, and arrested the cell-cycle, all of which suggests that compound 2j may represent a potentially useful start point to undergo further optimization toward a lead compound.
Keywords: Zanthoxylum nitidum; antileukemia activity; benzophenanthridine alkaloid derivatives; cell cycle and apoptosis; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
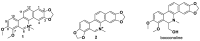

Similar articles
-
Insights on Antitumor Activity and Mechanism of Natural Benzophenanthridine Alkaloids.Molecules. 2023 Sep 13;28(18):6588. doi: 10.3390/molecules28186588. Molecules. 2023. PMID: 37764364 Free PMC article. Review.
-
Cytotoxicity of benzophenanthridine alkaloids from the roots of Zanthoxylum nitidum (Roxb.) DC. var. fastuosum How ex Huang.Nat Prod Res. 2015;29(14):1380-3. doi: 10.1080/14786419.2014.1002090. Epub 2015 Feb 3. Nat Prod Res. 2015. PMID: 25647513
-
A New Series of Antileukemic Agents: Design, Synthesis, In Vitro and In Silico Evaluation of Thiazole-Based ABL1 Kinase Inhibitors.Anticancer Agents Med Chem. 2021;21(9):1099-1109. doi: 10.2174/1871520620666200824100408. Anticancer Agents Med Chem. 2021. PMID: 32838725
-
Mild C(sp3)-H functionalization of dihydrosanguinarine and dihydrochelerythrine for development of highly cytotoxic derivatives.Eur J Med Chem. 2017 Sep 29;138:1-12. doi: 10.1016/j.ejmech.2017.06.021. Epub 2017 Jun 13. Eur J Med Chem. 2017. PMID: 28641156
-
Design, synthesis, in vitro antiproliferative activity and apoptosis-inducing studies of 1-(3',4',5'-trimethoxyphenyl)-3-(2'-alkoxycarbonylindolyl)-2-propen-1-one derivatives obtained by a molecular hybridisation approach.J Enzyme Inhib Med Chem. 2018 Dec;33(1):1225-1238. doi: 10.1080/14756366.2018.1493473. J Enzyme Inhib Med Chem. 2018. PMID: 30141353 Free PMC article.
Cited by
-
Insights on Antitumor Activity and Mechanism of Natural Benzophenanthridine Alkaloids.Molecules. 2023 Sep 13;28(18):6588. doi: 10.3390/molecules28186588. Molecules. 2023. PMID: 37764364 Free PMC article. Review.
-
Cytostatic Activity of Sanguinarine and a Cyanide Derivative in Human Erythroleukemia Cells Is Mediated by Suppression of c-MET/MAPK Signaling.Int J Mol Sci. 2023 Apr 30;24(9):8113. doi: 10.3390/ijms24098113. Int J Mol Sci. 2023. PMID: 37175820 Free PMC article.
References
-
- Juliusson G., Hough R. Leukemia. Prog. Tumor Res. 2015;43:87–100. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

