Link between Omega 3 Fatty Acids Carried by Lipoproteins and Breast Cancer Severity
- PMID: 35745191
- PMCID: PMC9230874
- DOI: 10.3390/nu14122461
Link between Omega 3 Fatty Acids Carried by Lipoproteins and Breast Cancer Severity
Abstract
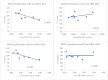
According to the International Agency for Research on Cancer (IARC) more than 10% of cancers can be explained by inadequate diet and excess body weight. Breast cancer is the most common cancer affecting women. The goal of our study is to clarify the relationship between ω3 fatty acids (FA) carried by different lipoproteins and breast cancer (BC) severity, according to two approaches: through clinic-biological data and through in vitro breast cancer cell models. The clinical study has been performed in sera from a cohort of BC women (n = 140, ICO, France) whose tumors differed by their hormone receptors status (HR− for tumors negative for estrogen receptors and progesterone receptors, HR+ for tumors positive for either estrogen receptors or progesterone receptors) and the level of proliferation markers (Ki-67 ≤ 20% Prolif− and Ki-67 ≥ 30% Prolif+). Lipids and ω3FA have been quantified in whole serum and in apoB-containing lipoproteins (Non-HDL) or free of it (HDL). Differences between Prolif− and Prolif+ were compared by Wilcoxon test in each sub-group HR+ and HR−. Results are expressed as median [25th−75th percentile]. Plasma cholesterol, triglycerides, HDL-cholesterol and Non-HDL cholesterol did not differ between Prolif− and Prolif+ sub-groups of HR− and HR+ patients. Plasma EPA and DHA concentrations did not differ either. In the HR− group, the distribution of EPA and DHA between HDL and Non-HDL differed significantly, as assessed by a higher ratio between the FA concentration in Non-HDL and HDL in Prolif− vs. Prolif+ patients (0.20 [0.15−0.36] vs. 0.04 [0.02−0.08], p = 0.0001 for EPA and 0.08 [0.04−0.10] vs. 0.04 [0.01−0.07], p = 0.04 for DHA). In this HR− group, a significant increase in Non-HDL EPA concentration was also observed in Prolif− vs. Prolif+ (0.18 [0.13−0.40] vs. 0.05 [0.02−0.07], p = 0.001). A relative enrichment on Non-HDL in EPA and DHA was also observed in Prolif− patients vs. Prolif+ patients, as assessed by a higher molar ratio between FA and apoB (0.12 [0.09−0.18] vs. 0.02 [0.01−0.05], p < 0.0001 for EPA and 1.00 [0.73−1.69 vs. 0.52 [0.14−1.08], p = 0.04 for DHA). These data were partly confirmed by an in vitro approach of proliferation of isolated lipoproteins containing EPA and DHA on MDA-MB-231 (HR−) and MCF-7 (HR+) cell models. Indeed, among all the studied fractions, only the correlation between the EPA concentration of Non-HDL was confirmed in vitro, although with borderline statistical significance (p = 0.07), in MDA-MB-231 cells. Non-HDL DHA, in the same cells model was significantly correlated to proliferation (p = 0.04). This preliminary study suggests a protective effect on breast cancer proliferation of EPA and DHA carried by apo B-containing lipoproteins (Non-HDL), limited to HR− tumors.
Keywords: DHA; EPA; HR−; breast cancer; lipoproteins; omega 3 PUFA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Eicosapentaenoic and Docosahexaenoic Acid Supplementation Increases HDL Content in n-3 Fatty Acids and Improves Endothelial Function in Hypertriglyceridemic Patients.Int J Mol Sci. 2023 Mar 11;24(6):5390. doi: 10.3390/ijms24065390. Int J Mol Sci. 2023. PMID: 36982461 Free PMC article. Clinical Trial.
-
Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: a review.Lipids Health Dis. 2017 Aug 10;16(1):149. doi: 10.1186/s12944-017-0541-3. Lipids Health Dis. 2017. PMID: 28797250 Free PMC article. Review.
-
The effect of dietary docosahexaenoic acid on plasma lipoproteins and tissue fatty acid composition in humans.Lipids. 1997 Nov;32(11):1137-46. doi: 10.1007/s11745-997-0146-5. Lipids. 1997. PMID: 9397398
-
Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men.Am J Clin Nutr. 2000 May;71(5):1085-94. doi: 10.1093/ajcn/71.5.1085. Am J Clin Nutr. 2000. PMID: 10799369 Clinical Trial.
-
Eicosapentaenoic Acid Versus Docosahexaenoic Acid as Options for Vascular Risk Prevention: A Fish Story.Am J Ther. 2016 May-Jun;23(3):e905-10. doi: 10.1097/MJT.0000000000000165. Am J Ther. 2016. PMID: 25828517 Review.
Cited by
-
Epigenetic Mechanisms of Endocrine-Disrupting Chemicals in Breast Cancer and Their Impact on Dietary Intake.J Xenobiot. 2024 Dec 24;15(1):1. doi: 10.3390/jox15010001. J Xenobiot. 2024. PMID: 39846533 Free PMC article. Review.
-
The Effects of Omega-3 Polyunsaturated Fatty Acids on Breast Cancer as a Preventive Measure or as an Adjunct to Conventional Treatments.Nutrients. 2023 Mar 7;15(6):1310. doi: 10.3390/nu15061310. Nutrients. 2023. PMID: 36986040 Free PMC article.
-
Link Between Metabolic Syndrome, Blood Lipid Markers, Dietary Lipids, and Survival in Women with Early-Stage Breast Cancer.Nutrients. 2024 Oct 22;16(21):3579. doi: 10.3390/nu16213579. Nutrients. 2024. PMID: 39519412 Free PMC article.
-
Lipid Metabolism and Relevance to Chronic Disease.Nutrients. 2025 Jun 5;17(11):1936. doi: 10.3390/nu17111936. Nutrients. 2025. PMID: 40507205 Free PMC article.
-
Fatty Acid Profiles in the Gonads of Red King Crab (Paralithodes camtschaticus) from the Barents Sea.Animals (Basel). 2023 Jan 17;13(3):336. doi: 10.3390/ani13030336. Animals (Basel). 2023. PMID: 36766225 Free PMC article.
References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C., Allen C., Barber R.M., Barregard L., Bhutta Z.A., Brenner H., Dicker D.J., Chimed-Orchir O., Dandona R., et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. - DOI - PMC - PubMed
-
- Agostoni C., Bresson J.L., Fairweather-Tait S. EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2010;8:1461. doi: 10.2903/j.efsa.2010.1461. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

