Eriodictyol Attenuates H2O2-Induced Oxidative Damage in Human Dermal Fibroblasts through Enhanced Capacity of Antioxidant Machinery
- PMID: 35745283
- PMCID: PMC9228723
- DOI: 10.3390/nu14122553
Eriodictyol Attenuates H2O2-Induced Oxidative Damage in Human Dermal Fibroblasts through Enhanced Capacity of Antioxidant Machinery
Abstract
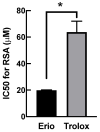
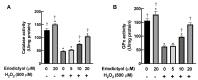
Oxidative stress in dermal fibroblasts is strongly correlated with the aging process of the skin. The application of natural compounds that can increase the ability of dermal fibroblasts to counteract oxidative stress is a promising approach to promote skin health and beauty. Eriodictyol is a flavonoid that exerts several pharmacological actions through its antioxidant properties. However, its protective effects on dermal fibroblasts have not yet been investigated. In this study, we investigated whether eriodictyol protects human dermal fibroblasts (BJ fibroblasts) from the harmful effects of hydrogen peroxide (H2O2). Eriodictyol pretreatment significantly prevented necrotic cell death caused by H2O2 exposure. In addition, the level of 2',7'-dichloro-dihydro-fluorescein oxidation was decreased, and that of glutathione was maintained, indicating that the beneficial effects of eriodictyol against H2O2 were closely associated with oxidative-stress attenuation. Eriodictyol mediates its antioxidant effects on dermal fibroblasts against H2O2 through (i) the direct neutralization of reactive oxygen species; (ii) the enhancement of the activities of H2O2-detoxifying enzymes, including catalase and glutathione peroxidase; and (iii) the induction of the expressions of catalase and glutathione peroxidase 1 via the activation of the Nrf2 signaling system. These results support the potential application of eriodictyol as an ingredient in skincare products for cosmeceutical and pharmaceutical purposes.
Keywords: antioxidants; eriodictyol; fibroblasts; hydrogen peroxide; oxidative stress; skin aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Pillai S., Oresajo C., Hayward J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

