In Vitro High-Throughput Toxicological Assessment of Nanoplastics
- PMID: 35745286
- PMCID: PMC9230863
- DOI: 10.3390/nano12121947
In Vitro High-Throughput Toxicological Assessment of Nanoplastics
Abstract
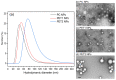
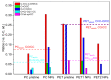
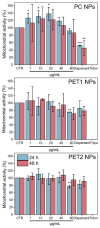
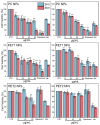
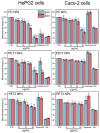
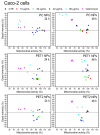
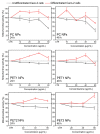
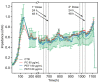
Sub-micrometer particles derived from the fragmentation of plastics in the environment can enter the food chain and reach humans, posing significant health risks. To date, there is a lack of adequate toxicological assessment of the effects of nanoplastics (NPs) in mammalian systems, particularly in humans. In this work, we evaluated the potential toxic effects of three different NPs in vitro: two NPs obtained by laser ablation (polycarbonate (PC) and polyethylene terephthalate (PET1)) and one (PET2) produced by nanoprecipitation. The physicochemical characterization of the NPs showed a smaller size, a larger size distribution, and a higher degree of surface oxidation for the particles produced by laser ablation. Toxicological evaluation performed on human cell line models (HePG2 and Caco-2) showed a higher toxic effect for the particles synthesized by laser ablation, with PC more toxic than PET. Interestingly, on differentiated Caco-2 cells, a conventional intestinal barrier model, none of the NPs produced toxic effects. This work wants to contribute to increase knowledge on the potential risks posed by NPs.
Keywords: cytotoxicity; high content screening; in vitro assays; laser ablation; nanoplastics; nanotoxicology; polycarbonate; polyethylene terephthalate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Plastic Europe—Association of Plastics Manufactures Plastics—The Facts 2021. [(accessed on 1 June 2022)]. Available online: file:///C:/Users/MDPI/Downloads/Plastics-the-Facts-2021-web-final.pdf.
-
- Oliveri Conti G., Ferrante M., Banni M., Favara C., Nicolosi I., Cristaldi A., Fiore M., Zuccarello P. Micro- and Nano-Plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population. Environ. Res. 2020;187:109677. doi: 10.1016/j.envres.2020.109677. - DOI - PubMed
LinkOut - more resources
Full Text Sources

