Effectiveness of the Novel Anti-TB Bedaquiline against Drug-Resistant TB in Africa: A Systematic Review of the Literature
- PMID: 35745490
- PMCID: PMC9229213
- DOI: 10.3390/pathogens11060636
Effectiveness of the Novel Anti-TB Bedaquiline against Drug-Resistant TB in Africa: A Systematic Review of the Literature
Abstract
Background: In 2018, an estimated 10.0 million people contracted tuberculosis (TB), and 1.5 million died from it, including 1.25 million HIV-negative persons and 251,000 HIV-associated TB fatalities. Drug-resistant tuberculosis (DR-TB) is an important contributor to global TB mortality. Multi-drug-resistant TB (MDR-TB) is defined as TB resistant to at least isoniazid (INH) and rifampin (RMP), which are recommended by the WHO as essential drugs for treatment.
Objective: To investigate the effectiveness of bedaquiline addition to the treatment of drug-resistant TB infections on the African continent.
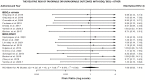
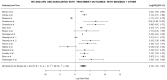
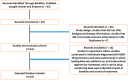
Methodology: The search engine databases Medline, PubMed, Google Scholar, and Embase were used to obtain published data pertaining to DR-TB between 2012 and 2021 in Africa. Included studies had to document clinical characteristics at treatment initiation and outcomes at the end of treatment (i.e., success, failure, recurrence, loss to follow-up, and death). The included studies were used to conduct a meta-analysis. All data analysis and visualization were performed using the R programming environment. The log risk ratios and sample variances were calculated for DR-TB patients treated with BBQ monotherapy vs. BDQ and other drug therapy. To quantify heterogeneity among the included studies, random effect sizes were calculated.
Results: A total of 16 studies in Africa from Mozambique (N = 1 study), Eswatini (N = 1 study), Democratic Republic of the Congo (N = 1 study), South Africa (N = 12 studies), and a multicenter study undertaken across Africa (N = 1 study) were included. In total, 22,368 individuals participated in the research studies. Among the patients, (55.2%; 12,350/22,368) were male while 9723/22,368 (44%) were female. Overall, (9%; 2033/22,368) of patients received BDQ monotherapy, while (88%; 19,630/22,368) patients received bedaquiline combined with other antibiotics. In total, (42%; 9465/22,368) of the patients were successfully treated. About (39%; 8653/22,368) of participants finished their therapy, meanwhile (5%; 1166/22,368) did not finish their therapy, while people (0.4%; 99/22,368) were lost to follow up. A total of (42%; 9265/22,368) patients died.
Conclusion: Very few studies on bedaquiline usage in DR-TB in Africa have been published to date. Bedaquiline has been shown to enhance DR-TB results in clinical studies and programmatic settings. Hence, the World Health Organization (WHO) has recommended that it be included in DR-TB regimens. However, in the current study limited improvement to DR-TB treatment results were observed using BDQ on the continent. Better in-country monitoring and reporting, as well as multi-country collaborative cohort studies of DR-TB, can expand the knowledge of bedaquiline usage and clinical impact, as well as the risks and benefits throughout the continent.
Keywords: Africa; bedaquiline (BDQ); drug-resistant TB; isoniazid (INH); rifampin (RMP); treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization . Rapid Communication: Key Changes to Treatment of Multidrug-and Rifampicin-Resistant Tuberculosis (MDR/RR-TB) World Health Organization; Geneva, Switzerland: 2018.
-
- Ndjeka N., Schnippel K., Master I., Meintjes G., Maartens G., Romero R., Padanilam X., Enwerem M., Chotoo S., Singh N. High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur. Respir. J. 2018;52:1801528. doi: 10.1183/13993003.01528-2018. - DOI - PubMed
-
- World Health Organization . Global Tuberculosis Report 2019: Fact Sheet. World Health Organization; Geneva, Switzerland: 2019. [(accessed on 15 December 2021)]. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

