Efficacy and Safety of Pathogen-Reduced Platelets Compared with Standard Apheresis Platelets: A Systematic Review of RCTs
- PMID: 35745493
- PMCID: PMC9231062
- DOI: 10.3390/pathogens11060639
Efficacy and Safety of Pathogen-Reduced Platelets Compared with Standard Apheresis Platelets: A Systematic Review of RCTs
Abstract
In this systematic review, we evaluate the efficacy and safety of blood components treated with pathogen reduction technologies (PRTs). We searched the Medline, Embase, Scopus, Ovid, and Cochrane Library to identify RCTs evaluating PRTs. Risk of bias assessment and the Mantel-Haenszel method for data synthesis were used. We included in this review 19 RCTs evaluating 4332 patients (mostly oncohematological patients) receiving blood components treated with three different PRTs. Compared with standard platelets (St-PLTs), the treatment with pathogen-reduced platelets (PR-PLTs) does not increase the occurrence of bleeding events, although a slight increase in the occurrence of severe bleeding events was observed in the overall comparison. No between-groups difference in the occurrence of serious adverse events was observed. PR-PLT recipients had a lower 1 and 24 h CI and CCI. The number of patients with platelet refractoriness and alloimmunization was significantly higher in PR-PLT recipients compared with St-PLT recipients. PR-PLT recipients had a higher number of platelet and RBC transfusions compared with St-PLT recipients, with a shorter transfusion time interval. The quality of evidence for these outcomes was from moderate to high. Blood components treated with PRTs are not implicated in serious adverse events, and PR-PLTs do not have a major effect on the increase in bleeding events. However, treatment with PRTs may require a greater number of transfusions in shorter time intervals and may be implicated in an increase in platelet refractoriness and alloimmunization.
Keywords: adverse events; alloimmunization; bleeding; pathogen inactivation; pathogen reduction technology; pathogen-reduced platelets; platelet count increment; refractoriness; systematic review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
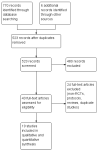









References
-
- Decreto del Ministero Della Salute 2 Novembre 2015. Disposizioni Relative ai Requisiti di Qualità e Sicurezza del Sangue e Degli Emocomponenti. Gazzetta Ufficiale n. 300—Suppl. Ordinario n. 69, 28 Dicembre 2015. [(accessed on 20 January 2022)]. Available online: https://www.gazzettaufficiale.it/eli/id/2015/12/28/15A09709/sg. (In Italian)
-
- Velati C., Romanò L., Pati I., Marano G., Piccinini V., Catalano L., Pupella S., Vaglio S., Veropalumbo E., Masiello F., et al. Prevalence, incidence and residual risk of transfusion-transmitted hepatitis B virus infection in Italy from 2009 to 2018. Blood Transfus. 2019;17:409–417. doi: 10.2450/2019.0245-19. - DOI - PMC - PubMed
-
- Velati C., Romanò L., Piccinini V., Marano G., Catalano L., Pupella S., Facco G., Pati I., Tosti M.E., Vaglio S., et al. Prevalence, incidence and residual risk of transfusion-transmitted hepatitis C virus and human immunodeficiency virus after the implementation of nucleic acid testing in Italy: A 7-year (2009–2015) survey. Blood Transfus. 2018;16:422–432. doi: 10.2450/2018.0069-18. - DOI - PMC - PubMed
-
- Italian National Blood Centre . Linea Guida per la Prevenzione Della Contaminazione Batterica del Sangue Intero e Degli Emocomponenti. Italian National Blood Centre; Rome, Italy: 2008. Linee Guida CNS 02 del 07.07.2008.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

