Applications in Which Aptamers Are Needed or Wanted in Diagnostics and Therapeutics
- PMID: 35745612
- PMCID: PMC9228505
- DOI: 10.3390/ph15060693
Applications in Which Aptamers Are Needed or Wanted in Diagnostics and Therapeutics
Abstract
One strategy for bringing aptamers more into the mainstream of biomedical diagnostics and therapeutics is to exploit niche applications where aptamers are truly needed or wanted for their innate differences versus antibodies. This brief review article highlights some of those relatively rare applications in which aptamers are necessary or better suited to the user requirements than antibodies with explanations for why the aptamer is a necessary or superior choice. These situations include when no commercial antibody exists, when antibodies are excessively difficult to develop against a particular target because the target is highly toxic to host animals, when antibodies fail to discriminate closely related targets, when a smaller size is preferable to penetrate a tissue, when humanized monoclonal antibodies are too expensive and when the target is rapidly evolving or mutating. Examples of each are provided to illustrate these points.
Keywords: Cyclospora; SELEX; aptamer; diagnostic; humanized; liver fluke; monoclonal antibody; reproducibility; therapeutic; toxic.
Conflict of interest statement
The author claims no conflict of interest.
Figures
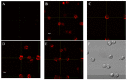
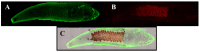
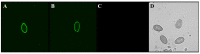

Similar articles
-
Predicting the Uncertain Future of Aptamer-Based Diagnostics and Therapeutics.Molecules. 2015 Apr 16;20(4):6866-87. doi: 10.3390/molecules20046866. Molecules. 2015. PMID: 25913927 Free PMC article. Review.
-
Recent developments in cell-SELEX technology for aptamer selection.Biochim Biophys Acta Gen Subj. 2018 Oct;1862(10):2323-2329. doi: 10.1016/j.bbagen.2018.07.029. Epub 2018 Jul 27. Biochim Biophys Acta Gen Subj. 2018. PMID: 30059712 Review.
-
Advances of aptamers screened by Cell-SELEX in selection procedure, cancer diagnostics and therapeutics.Anal Biochem. 2020 Jun 1;598:113620. doi: 10.1016/j.ab.2020.113620. Epub 2020 Feb 19. Anal Biochem. 2020. PMID: 32087127 Review.
-
Replacing antibodies with aptamers in lateral flow immunoassay.Biosens Bioelectron. 2015 Sep 15;71:230-242. doi: 10.1016/j.bios.2015.04.041. Epub 2015 Apr 14. Biosens Bioelectron. 2015. PMID: 25912679 Review.
-
Trends in aptamer selection methods and applications.Analyst. 2015 Aug 21;140(16):5379-99. doi: 10.1039/c5an00954e. Analyst. 2015. PMID: 26114391 Review.
Cited by
-
Evolution of complexity in non-viral oligonucleotide delivery systems: from gymnotic delivery through bioconjugates to biomimetic nanoparticles.RNA Biol. 2022 Jan;19(1):1256-1275. doi: 10.1080/15476286.2022.2147278. RNA Biol. 2022. PMID: 36411594 Free PMC article. Review.
-
Cyclospora cayetanensis: A Perspective (2020-2023) with Emphasis on Epidemiology and Detection Methods.Microorganisms. 2023 Aug 28;11(9):2171. doi: 10.3390/microorganisms11092171. Microorganisms. 2023. PMID: 37764015 Free PMC article.
-
Aptameric Fluorescent Biosensors for Liver Cancer Diagnosis.Biosensors (Basel). 2023 Jun 4;13(6):617. doi: 10.3390/bios13060617. Biosensors (Basel). 2023. PMID: 37366982 Free PMC article. Review.
-
Aptamers' Potential to Fill Therapeutic and Diagnostic Gaps.Pharmaceuticals (Basel). 2024 Jan 12;17(1):105. doi: 10.3390/ph17010105. Pharmaceuticals (Basel). 2024. PMID: 38256938 Free PMC article.
-
Global health perspectives on antibacterial drug discovery and the preclinical pipeline.Nat Rev Microbiol. 2025 Aug;23(8):474-490. doi: 10.1038/s41579-025-01167-w. Epub 2025 Mar 27. Nat Rev Microbiol. 2025. PMID: 40148602 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

