Mepirapim, a Novel Synthetic Cannabinoid, Induces Addiction-Related Behaviors through Neurochemical Maladaptation in the Brain of Rodents
- PMID: 35745629
- PMCID: PMC9229951
- DOI: 10.3390/ph15060710
Mepirapim, a Novel Synthetic Cannabinoid, Induces Addiction-Related Behaviors through Neurochemical Maladaptation in the Brain of Rodents
Abstract
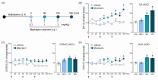
Mepirapim is a synthetic cannabinoid that has recently been abused for recreational purposes. Although serious side effects have been reported from users, the dangerous pharmacological effects of Mepirapim have not been scientifically demonstrated. In this study, we investigated the addictive potential of Mepirapim through an intravenous self-administration test and a conditioned place preference test in rodents. Moreover, to determine whether the pharmacological effects of Mepirapim are mediated by cannabinoid receptors, we investigated whether Mepirapim treatment induces cannabinoid tetrad symptoms in mice. Lastly, to identify Mepirapim induced neurochemical maladaptation in the brains of mice, we performed microdialysis, western blots and neurotransmitter enzyme-linked immunosorbent assays. In the results, Mepirapim supported the maintenance of intravenous self-administration and the development of conditioned place preference. As a molecular mechanism of Mepirapim addiction, we identified a decrease in GABAeric signalling and an increase in dopaminergic signalling in the brain reward circuit. Finally, by confirming the Mepirapim-induced expression of cannabinoid tetrad symptoms, we confirmed that Mepirapim acts pharmacologically through cannabinoid receptor one. Taken together, we found that Mepirapim induces addiction-related behaviours through neurochemical maladaptation in the brain. On the basis of these findings, we propose the strict regulation of recreational abuse of Mepirapim.
Keywords: Mepirapim; addiction; dopamine; synthetic cannabinoid; γ-aminobutyric acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- UNODC . World Drug Report 2021. 3rd ed. United Nations Office on Drugs and Crime; Vienna, Austria: 2021. p. 121.
-
- UNODC . Current NPS Threats. United Nations Office on Drugs and Crime Early Warning Advisory; Vienna, Austria: 2021. p. 6.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

