A Comparative Study of the Pharmaceutical Properties between Amorphous Drugs Loaded-Mesoporous Silica and Pure Amorphous Drugs Prepared by Solvent Evaporation
- PMID: 35745649
- PMCID: PMC9228546
- DOI: 10.3390/ph15060730
A Comparative Study of the Pharmaceutical Properties between Amorphous Drugs Loaded-Mesoporous Silica and Pure Amorphous Drugs Prepared by Solvent Evaporation
Abstract
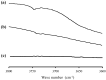
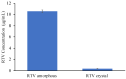
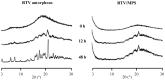
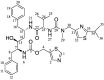
The formulation of poorly water-soluble drugs is one of the main challenges in the pharmaceutical industry, especially in the development of oral dosage forms. Meanwhile, there is an increase in the number of poorly soluble drugs that have been discovered as new chemical entities. It was also reported that the physical transformation of a drug from a crystalline form into an amorphous state could be used to increase its solubility. Therefore, this study aims to evaluate the pharmaceutical properties of amorphous drug loaded-mesoporous silica (MPS) and pure amorphous drugs. Ritonavir (RTV) was used as a model of a poorly water-soluble drug due to its low recrystallization tendency. RTV loaded-MPS (RTV/MPS) and RTV amorphous were prepared using the solvent evaporation method. Based on observation, a halo pattern in the powder X-ray diffraction pattern and a single glass transition (Tg) in the modulated differential scanning calorimetry (MDSC) curve was discovered in RTV amorphous, indicating its amorphization. The Tg was not detected in RTV/MPS, which showed that the loading RTV was completed. The solid-state NMR and FT-IR spectroscopy also showed the interaction between RTV and the surface of MPS in the mesopores. The high supersaturation of RTV was not achieved for both RTV/MPS and the amorphous state due to its strong interaction with the surface of MPS and was not properly dispersed in the medium, respectively. In the dissolution test, the molecular dispersion of RTV within MPS caused rapid dissolution at the beginning, while the amorphous showed a low rate due to its agglomeration. The stability examination showed that the loading process significantly improved the physical and chemical stability of RTV amorphous. These results indicated that the pharmaceutical properties of amorphous drugs could be improved by loaded-MPS.
Keywords: amorphous; mesoporous silica; pharmaceutical properties; ritonavir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Skorupska E., Jeziorna A., Potrzebowski M.J. Thermal solvent-free method of loading of pharmaceutical cocrystals into the pores of silica particles: A Case of naproxen/picolinamide cocrystal. J. Phys. Chem. C. 2016;120:13169–13180. doi: 10.1021/acs.jpcc.6b05302. - DOI
-
- Zhao Z., Katai H., Higashi K., Ueda K., Kawakami K., Moribe K. Cryo-TEM and AFM Observation of the Time-Dependent Evolution of Amorphous Probucol Nanoparticles Formed by the Aqueous Dispersion of Ternary Solid Dispersions. Mol. Pharm. 2019;16:2184–2198. doi: 10.1021/acs.molpharmaceut.9b00158. - DOI - PubMed
-
- Skorupska E., Paluch P., Jeziorna A., Potrzebowski M.J. NMR study of BA/FBA cocrystal confined within mesoporous silica nanoparticles employing thermal solid phase transformation. J. Phys. Chem. C. 2015;119:8652–8661. doi: 10.1021/jp5123008. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

