Influence of Oil Polarity and Cosurfactants on the Foamability of Mono- and Diacylphosphatidylcholine Stabilized Emulsions
- PMID: 35745785
- PMCID: PMC9230088
- DOI: 10.3390/pharmaceutics14061212
Influence of Oil Polarity and Cosurfactants on the Foamability of Mono- and Diacylphosphatidylcholine Stabilized Emulsions
Abstract
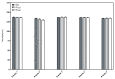
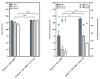
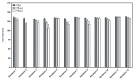
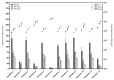
Foam formulations are safe and effective therapy options for the treatment of chronic skin conditions that require the application of a topical formulation to delicate skin areas, such as scalp psoriasis or seborrheic dermatitis. This study focused on the development of foamable emulsions based on aqueous phospholipid blends. The effects of cosurfactants (nonionic Lauryglucoside (LG); zwitterionic Lauramidopropyl betaine (LAPB)), as well as of oil phases of different polarities, namely paraffin oil (PO), medium-chain triglycerides (MCT) and castor oil (CO), were investigated. The foaming experiments showed that both the type of cosurfactant, as well as the type of oil phase, affects the quality of the resulting foam. Emulsions that were based on a combination of hydrogenated lysophosphatidylcholine (hLPC) and a non-hydrogenated phospholipid, as well as LG as a cosurfactant and MCT as an oil phase, yielded the most satisfactory results. Furthermore, profile analysis tensiometry (PAT), polarization microscopy and laser diffraction analysis were used to characterize the developed formulations. These experiments suggest that the employed phospholipids predominantly stabilize the emulsions, while the cosurfactants are mainly responsible for the formation and stabilization of the foams. However, it appears that both sets of excipients are needed in order to acquire stable emulsions with satisfactory foaming properties.
Keywords: diacylphosphatidylcholine; emulsion; foam; laser diffraction measurements; monoacylphosphatidylcholine; phospholipids; profile analysis tensiometry (PAT).
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Arzhavitina A. Ph.D. Thesis. Christian Albrecht University; Kiel, Germany: 2009. Foams as Novel Delivery Vehicle for Topical Application.
-
- Purdon C.H., Haigh J.M., Surber C., Smith E.W. Foam Drug Delivery in Dermatology. Am. J. Drug Deliv. 2003;1:71–75. doi: 10.2165/00137696-200301010-00006. - DOI
-
- Shinde N. Pharmaceutical Foam Drug Delivery System: General Considerations. Indo Am. J. Pharm. Res. 2013;3:1322–1327.
LinkOut - more resources
Full Text Sources

