HO-3867 Induces Apoptosis via the JNK Signaling Pathway in Human Osteosarcoma Cells
- PMID: 35745828
- PMCID: PMC9229449
- DOI: 10.3390/pharmaceutics14061257
HO-3867 Induces Apoptosis via the JNK Signaling Pathway in Human Osteosarcoma Cells
Abstract
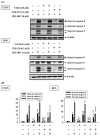
Metastatic osteosarcoma often results in poor prognosis despite the application of surgical en bloc excision along with chemotherapy. HO-3867 is a curcumin analog that induces cell apoptosis in several cancers, but the apoptotic effect and its mechanisms on osteosarcoma cells are still unknown. After observing the decrease in cellular viability of three human osteosarcoma U2OS, HOS, and MG-63 cell lines, and the induction of cellular apoptosis and arrest in sub-G1 phase in U2OS and HOS cells by HO-3867, the human apoptosis array showed that heme oxygenase (HO)-1 and cleaved caspase-3 expressions had significant increases after HO-3867 treatment in U2OS cells and vice versa for cellular inhibitors of apoptosis (cIAP)1 and X-chromosome-linked IAP (XIAP). Western blot analysis verified the results and showed that HO-3867 activated the initiators of both extrinsic caspase 8 and intrinsic caspase 9, and significantly increased cleaved PARP expression in U2OS and HOS cells. Moreover, with the addition of HO-3867, ERK1/2, and JNK1/2 phosphorylation were increased in U2OS and HOS cells. Using the inhibitor of JNK (JNK in 8), HO-3867's increases in cleaved caspases 3, 8, and 9 could be expectedly suppressed, indicating that JNK signaling is responsible for both apoptotic pathways, including extrinsic and intrinsic, in U2OS and HOS cells caused by HO-3867. Through JNK signaling, HO-3867 has proven to be effective in causing both extrinsic and intrinsic apoptotic pathways of human osteosarcoma cells.
Keywords: ERK; HO-3867; JNK; apoptosis; curcumin; osteosarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ottaviani G., Jaffe N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009;152:3–13. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

