PLGA Carriers for Controlled Release of Levofloxacin in Anti-Tuberculosis Therapy
- PMID: 35745846
- PMCID: PMC9227258
- DOI: 10.3390/pharmaceutics14061275
PLGA Carriers for Controlled Release of Levofloxacin in Anti-Tuberculosis Therapy
Abstract
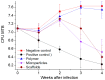
Levofloxacin (LFX) is a highly effective anti-tuberculosis drug with a pronounced bactericidal activity against Mycobacterium tuberculosis (Mtb). In this work, an "organic solvent-free" approach has been used for the development of polylactic-co-glycolic acid (PLGA) microparticles and scaffolds containing LFX at a therapeutically significant concentration, providing for its sustained release. To achieve the target, both nonpolar supercritical carbon dioxide and polar supercritical trifluoromethane have been used. By changing the composition, surface morphology, size, and internal structure of the polymer carriers, one can control the kinetics of the LFX release into phosphate buffered saline solutions and physiological media, providing for its acceptable burst and desirable concentration in the prolonged phase. The biocompatibility and bactericidal efficacy of PLGA/LFX carriers assessed both in vitro (against Mtb phagocytosed by macrophages) and in vivo (against inbred BALB/c mice aerogenically infected with Mtb) demonstrated their anti-tuberculosis activity comparable with that of the standard daily intragastric levofloxacin administration. These results make it possible to consider the developed compositions as a promising candidate for anti-tuberculosis control release formulations providing for the further evaluation of their activity against Mtb and their metabolism in vivo over long periods of tuberculosis infection.
Keywords: PLGA microparticles and scaffolds; anti-tuberculosis drugs; levofloxacin; supercritical fluid technologies; sustained release formulations.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources

