A Historical Review of Brain Drug Delivery
- PMID: 35745855
- PMCID: PMC9229021
- DOI: 10.3390/pharmaceutics14061283
A Historical Review of Brain Drug Delivery
Abstract
The history of brain drug delivery is reviewed beginning with the first demonstration, in 1914, that a drug for syphilis, salvarsan, did not enter the brain, due to the presence of a blood-brain barrier (BBB). Owing to restricted transport across the BBB, FDA-approved drugs for the CNS have been generally limited to lipid-soluble small molecules. Drugs that do not cross the BBB can be re-engineered for transport on endogenous BBB carrier-mediated transport and receptor-mediated transport systems, which were identified during the 1970s-1980s. By the 1990s, a multitude of brain drug delivery technologies emerged, including trans-cranial delivery, CSF delivery, BBB disruption, lipid carriers, prodrugs, stem cells, exosomes, nanoparticles, gene therapy, and biologics. The advantages and limitations of each of these brain drug delivery technologies are critically reviewed.
Keywords: IgG fusion proteins; blood–brain barrier; carrier-mediated transport; endothelium; genetic engineering; liposomes; nanoparticles; receptor-mediated transport.
Conflict of interest statement
W.M.P. is an inventor of patents on the delivery of biologics to brain.
Figures




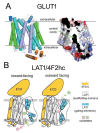

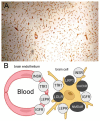


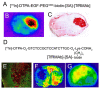
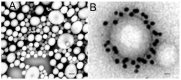


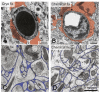
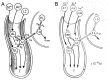



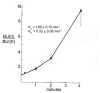

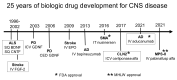
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

