Novel In Situ-Cross-Linked Electrospun Gelatin/Hydroxyapatite Nonwoven Scaffolds Prove Suitable for Periodontal Tissue Engineering
- PMID: 35745858
- PMCID: PMC9230656
- DOI: 10.3390/pharmaceutics14061286
Novel In Situ-Cross-Linked Electrospun Gelatin/Hydroxyapatite Nonwoven Scaffolds Prove Suitable for Periodontal Tissue Engineering
Abstract
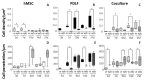
Periodontal diseases affect millions of people worldwide and can result in tooth loss. Regenerative treatment options for clinical use are thus needed. We aimed at developing new nonwoven-based scaffolds for periodontal tissue engineering. Nonwovens of 16% gelatin/5% hydroxyapatite were produced by electrospinning and in situ glyoxal cross-linking. In a subset of scaffolds, additional porosity was incorporated via extractable polyethylene glycol fibers. Cell colonization and penetration by human mesenchymal stem cells (hMSCs), periodontal ligament fibroblasts (PDLFs), or cocultures of both were visualized by scanning electron microscopy and 4',6-diamidin-2-phenylindole (DAPI) staining. Metabolic activity was assessed via Alamar Blue® staining. Cell type and differentiation were analyzed by immunocytochemical staining of Oct4, osteopontin, and periostin. The electrospun nonwovens were efficiently populated by both hMSCs and PDLFs, while scaffolds with additional porosity harbored significantly more cells. The metabolic activity was higher for cocultures of hMSCs and PDLFs, or for PDLF-seeded scaffolds. Periostin and osteopontin expression was more pronounced in cocultures of hMSCs and PDLFs, whereas Oct4 staining was limited to hMSCs. These novel in situ-cross-linked electrospun nonwoven scaffolds allow for efficient adhesion and survival of hMSCs and PDLFs. Coordinated expression of differentiation markers was observed, which rendered this platform an interesting candidate for periodontal tissue engineering.
Keywords: biocompatible materials; gelatin; hydroxyapatites; mesenchymal stem cells; periodontal guided tissue regeneration; periodontal ligament; periodontitis; regenerative medicine; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Periodontal Ligament-Mimetic Fibrous Scaffolds Regulate YAP-Associated Fibroblast Behaviors and Promote Regeneration of Periodontal Defect in Relation to the Scaffold Topography.ACS Appl Mater Interfaces. 2023 Jan 11;15(1):599-616. doi: 10.1021/acsami.2c18893. Epub 2022 Dec 28. ACS Appl Mater Interfaces. 2023. PMID: 36575925 Free PMC article.
-
Tailoring surface nanoroughness of electrospun scaffolds for skeletal tissue engineering.Acta Biomater. 2017 Sep 1;59:82-93. doi: 10.1016/j.actbio.2017.07.003. Epub 2017 Jul 6. Acta Biomater. 2017. PMID: 28690010
-
Periodontal regeneration with stem cells-seeded collagen-hydroxyapatite scaffold.J Biomater Appl. 2016 Jul;31(1):121-31. doi: 10.1177/0885328216637978. Epub 2016 Mar 22. J Biomater Appl. 2016. PMID: 27009932
-
Role of periodontal ligament fibroblasts in periodontitis: pathological mechanisms and therapeutic potential.J Transl Med. 2024 Dec 21;22(1):1136. doi: 10.1186/s12967-024-05944-8. J Transl Med. 2024. PMID: 39709490 Free PMC article. Review.
-
Biomaterials for periodontal regeneration: a review of ceramics and polymers.Biomatter. 2012 Oct-Dec;2(4):271-7. doi: 10.4161/biom.22948. Biomatter. 2012. PMID: 23507891 Free PMC article. Review.
Cited by
-
Direction-oriented fiber guiding with a tunable tri-layer-3D scaffold for periodontal regeneration.RSC Adv. 2024 Jun 19;14(28):19806-19822. doi: 10.1039/d4ra01459f. eCollection 2024 Jun 18. RSC Adv. 2024. PMID: 38899033 Free PMC article.
-
Electrospun Materials for Biomedical Applications.Pharmaceutics. 2022 Jul 26;14(8):1556. doi: 10.3390/pharmaceutics14081556. Pharmaceutics. 2022. PMID: 35893812 Free PMC article.
-
Nanomaterials in Scaffolds for Periodontal Tissue Engineering: Frontiers and Prospects.Bioengineering (Basel). 2022 Sep 1;9(9):431. doi: 10.3390/bioengineering9090431. Bioengineering (Basel). 2022. PMID: 36134977 Free PMC article. Review.
-
Recent Advances on Electrospun Nanofibers for Periodontal Regeneration.Nanomaterials (Basel). 2023 Apr 7;13(8):1307. doi: 10.3390/nano13081307. Nanomaterials (Basel). 2023. PMID: 37110894 Free PMC article. Review.
-
Hierarchical Biomaterial Scaffolds for Periodontal Tissue Engineering: Recent Progress and Current Challenges.Int J Mol Sci. 2024 Aug 6;25(16):8562. doi: 10.3390/ijms25168562. Int J Mol Sci. 2024. PMID: 39201249 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

