Electrospinning-Generated Nanofiber Scaffolds Suitable for Integration of Primary Human Circulating Endothelial Progenitor Cells
- PMID: 35746031
- PMCID: PMC9229005
- DOI: 10.3390/polym14122448
Electrospinning-Generated Nanofiber Scaffolds Suitable for Integration of Primary Human Circulating Endothelial Progenitor Cells
Abstract
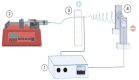
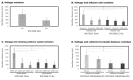
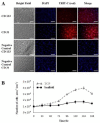
The extracellular matrix is fundamental in order to maintain normal function in many organs such as the blood vessels, heart, liver, or bones. When organs fail or experience injury, tissue engineering and regenerative medicine elicit the production of constructs resembling the native extracellular matrix, supporting organ restoration and function. In this regard, is it possible to optimize structural characteristics of nanofiber scaffolds obtained by the electrospinning technique? This study aimed to produce partially degraded collagen (gelatin) nanofiber scaffolds, using the electrospinning technique, with optimized parameters rendering different morphological characteristics of nanofibers, as well as assessing whether the resulting scaffolds are suitable to integrate primary human endothelial progenitor cells, obtained from peripheral blood with further in vitro cell expansion. After different assay conditions, the best nanofiber morphology was obtained with the following electrospinning parameters: 15 kV, 0.06 mL/h, 1000 rpm and 12 cm needle-to-collector distance, yielding an average nanofiber thickness of 333 ± 130 nm. Nanofiber scaffolds rendered through such electrospinning conditions were suitable for the integration and proliferation of human endothelial progenitor cells.
Keywords: endothelial cells; nanofiber scaffolds; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Electrospun Nanofiber Scaffolds and Their Hydrogel Composites for the Engineering and Regeneration of Soft Tissues.Methods Mol Biol. 2017;1570:261-278. doi: 10.1007/978-1-4939-6840-4_18. Methods Mol Biol. 2017. PMID: 28238143
-
Mass production of nanofibrous extracellular matrix with controlled 3D morphology for large-scale soft tissue regeneration.Tissue Eng Part C Methods. 2013 Jun;19(6):458-72. doi: 10.1089/ten.TEC.2012.0417. Epub 2012 Dec 12. Tissue Eng Part C Methods. 2013. PMID: 23102268 Clinical Trial.
-
A comparison of nanoscale and multiscale PCL/gelatin scaffolds prepared by disc-electrospinning.Colloids Surf B Biointerfaces. 2016 Oct 1;146:632-41. doi: 10.1016/j.colsurfb.2016.07.009. Epub 2016 Jul 7. Colloids Surf B Biointerfaces. 2016. PMID: 27429297
-
In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: Innovative nanofiber designs, stem cell approaches, and future perspectives.J Biomed Mater Res A. 2022 Feb;110(2):443-461. doi: 10.1002/jbm.a.37290. Epub 2021 Aug 13. J Biomed Mater Res A. 2022. PMID: 34390324 Review.
-
Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering.Tissue Eng Regen Med. 2017 Aug 10;14(6):699-718. doi: 10.1007/s13770-017-0075-9. eCollection 2017 Dec. Tissue Eng Regen Med. 2017. PMID: 30603521 Free PMC article. Review.
Cited by
-
Endothelial progenitor cells for fabrication of engineered vascular units and angiogenesis induction.Cell Prolif. 2024 Sep;57(9):e13716. doi: 10.1111/cpr.13716. Epub 2024 Jul 25. Cell Prolif. 2024. PMID: 39051852 Free PMC article. Review.
-
Three-Dimensional Printer-Assisted Electrospinning for Fabricating Intricate Biological Tissue Mimics.Nanomaterials (Basel). 2023 Nov 8;13(22):2913. doi: 10.3390/nano13222913. Nanomaterials (Basel). 2023. PMID: 37999268 Free PMC article.
-
Therapeutic Potential of Endothelial Progenitor Cells in Angiogenesis and Cardiac Regeneration: A Systematic Review and Meta-Analysis of Rodent Models.Adv Pharm Bull. 2025 Jun 16;15(2):268-283. doi: 10.34172/apb.025.45122. eCollection 2025 Jul. Adv Pharm Bull. 2025. PMID: 40922747 Free PMC article. Review.
-
Electrospun Nanomaterials Based on Cellulose and Its Derivatives for Cell Cultures: Recent Developments and Challenges.Polymers (Basel). 2023 Feb 26;15(5):1174. doi: 10.3390/polym15051174. Polymers (Basel). 2023. PMID: 36904415 Free PMC article. Review.
-
Beyond Vision: An Overview of Regenerative Medicine and Its Current Applications in Ophthalmological Care.Cells. 2024 Jan 17;13(2):179. doi: 10.3390/cells13020179. Cells. 2024. PMID: 38247870 Free PMC article. Review.
References
-
- Ahmed M.K., Moydeen A.M., Ismail A.M., El-Naggar M.E., Menazea A.A., El-Newehy M.H. Wound dressing properties of functionalized environmentally biopolymer loaded with selenium nanoparticles. J. Mol. Struct. 2021;1225:129138. doi: 10.1016/j.molstruc.2020.129138. - DOI
-
- Salingova B., Madarasova M., Stejskal S., Tesarova L., Simara P., Koutna I. From Endothelial Progenitor Cells to Tissue Engineering: How Far have we Come? J. Stem Cell Res. Ther. 2014;1:1.
LinkOut - more resources
Full Text Sources

