Antibody Response in Healthcare Workers before and after the Third Dose of Anti-SARS-CoV-2 Vaccine: A Pilot Study
- PMID: 35746470
- PMCID: PMC9229040
- DOI: 10.3390/vaccines10060862
Antibody Response in Healthcare Workers before and after the Third Dose of Anti-SARS-CoV-2 Vaccine: A Pilot Study
Abstract
The SARS-CoV-2 pandemic led to the development of various vaccines. The BNT162b2 mRNA vaccine was the first approved due to its efficacy in eliciting a humoral immunity response after the second dose. However, a decrease in the antibody concentration was observed over time. Therefore, the administration of a third dose was scheduled, primarily for frail people and workers of essential public activities. The aim of this study was to assess the level of antibodies against the spike (S) RBD of SARS-CoV-2 in healthcare workers before and after the third dose of BNT162b2 vaccine, according to sex, age, and the time interval between vaccine doses and tests. All 37 (12 males, 25 females, 19 < 50 years old, 18 ≥ 50 years old) healthcare workers recruited showed a consistent antibody titer increase after the third dose. Data analysis showed that the antibody concentration before the third dose significantly decreased as the time interval up to the test increased, and a significantly higher level was shown in young than older people. Cluster analysis revealed that young females had a higher antibody level than older females before the third dose (p < 0.05). This study indicated the benefit of the third dose of BNT162b2 vaccine and its effect on leveling up the humoral immune response.
Keywords: BNT162b2 mRNA vaccine; SARS-CoV-2; booster dose; healthcare workers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

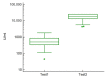


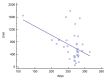
References
-
- Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., Brooks N., Smaja M., Mircus G., Pan K., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. - DOI - PMC - PubMed
-
- Thomas S.J., Moreira E.D., Jr., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Polack F.P., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. - DOI - PMC - PubMed
-
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

