Evaluation of a Lipopolysaccharide and Resiquimod Combination as an Adjuvant with Inactivated Newcastle Disease Virus Vaccine in Chickens
- PMID: 35746503
- PMCID: PMC9229813
- DOI: 10.3390/vaccines10060894
Evaluation of a Lipopolysaccharide and Resiquimod Combination as an Adjuvant with Inactivated Newcastle Disease Virus Vaccine in Chickens
Abstract
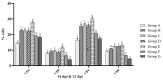
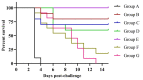
Various toll-like receptor (TLR) agonists have shown potential as adjuvants with different vaccines in both human and livestock species, including chickens. Our previous studies on combination of lipopolysaccharide (LPS; TLR4 agonist) and resiquimod (R-848; TLR7 agonist) showed the synergistic up-regulation of pro-inflammatory Th1 and Th2 cytokines in chicken peripheral blood mononuclear cells (PMBCs). Hence, the present study aimed to explore the combined adjuvant effect of LPS and R-848 with inactivated Newcastle disease virus (NDV) vaccine in chickens. Two weeks-old SPF chickens were immunized with inactivated NDV vaccine along with a combination of LPS and R-848 as an adjuvant with suitable control groups. A booster dose was given two weeks later. Antibody responses were assessed by enzyme linked immunosorbent assay (ELISA) and hemagglutination inhibition (HI) test, while cell-mediated immune responses were analyzed by a lymphocyte transformation test (LTT) and flow cytometry following vaccination. Two weeks post-booster, the birds were challenged with a velogenic strain of NDV, and protection against clinical signs, mortality and virus shedding was analyzed. The results indicated that inactivated NDV vaccine with R-848 induced significantly higher humoral and cellular immune responses with 100% protection against mortality and viral shedding following a virulent NDV challenge. However, the combination of LPS and R-848 along with inactivated NDV vaccine produced poor humoral and cellular immune responses and could not afford protection against challenge infection and virus shedding when compared to the vaccine-alone group, indicating the deleterious effects of the combination on antigen-specific immune responses. In conclusion, the combination of LPS and R-848 showed the inhibitory effects on antigen-specific humoral, cellular and protective immune responses when used as an adjuvant with inactivated NDV vaccines in chickens. This inhibitory effect might have occurred due to systemic cytokine storm. A nanoparticle-based delivery of the combination of LPS and R-848 for slow and sustained release could be tried as an alternative method to explore the synergistic effect of the combination as an adjuvant in chickens.
Keywords: LPS; NDV; TLR4; TLR7; adjuvant; combination; resiquimod.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Adjuvant potential of resiquimod with inactivated Newcastle disease vaccine and its mechanism of action in chicken.Vaccine. 2015 Aug 26;33(36):4526-32. doi: 10.1016/j.vaccine.2015.07.016. Epub 2015 Jul 17. Vaccine. 2015. PMID: 26192354
-
Co-administration of toll-like receptor (TLR)-3 and 4 ligands augments immune response to Newcastle disease virus (NDV) vaccine in chicken.Vet Res Commun. 2019 Nov;43(4):225-230. doi: 10.1007/s11259-019-09763-x. Epub 2019 Aug 24. Vet Res Commun. 2019. PMID: 31446518
-
Cytokine expression, protection and shedding reduction induced by the combination of lipopolysaccharide with Montanoid ISA71 in oil-based Newcastle disease vaccine.Microb Pathog. 2024 Mar;188:106542. doi: 10.1016/j.micpath.2024.106542. Epub 2024 Jan 9. Microb Pathog. 2024. PMID: 38199445
-
Comparative efficacy of commercial inactivated Newcastle disease virus vaccines against Newcastle disease virus genotype VII in broiler chickens.Poult Sci. 2019 May 1;98(5):2000-2007. doi: 10.3382/ps/pey559. Poult Sci. 2019. PMID: 30561723
-
A Booster with a Genotype-Matched Inactivated Newcastle Disease Virus (NDV) Vaccine Candidate Provides Better Protection against a Virulent Genotype XIII.2 Virus.Vaccines (Basel). 2023 May 20;11(5):1005. doi: 10.3390/vaccines11051005. Vaccines (Basel). 2023. PMID: 37243108 Free PMC article.
Cited by
-
Toll-like Receptor Ligands Enhance Vaccine Efficacy against a Virulent Newcastle Disease Virus Challenge in Chickens.Pathogens. 2023 Oct 11;12(10):1230. doi: 10.3390/pathogens12101230. Pathogens. 2023. PMID: 37887747 Free PMC article.
-
Structure of the Newcastle Disease Virus L protein in complex with tetrameric phosphoprotein.Nat Commun. 2023 Mar 10;14(1):1324. doi: 10.1038/s41467-023-37012-y. Nat Commun. 2023. PMID: 36898997 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

