Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real-World Estimation of Safety Profile
- PMID: 35746532
- PMCID: PMC9228257
- DOI: 10.3390/vaccines10060924
Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real-World Estimation of Safety Profile
Abstract
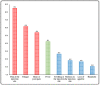
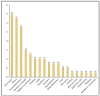
Vaccines are considered to be the most beneficial means for combating the COVID-19 pandemic. Although vaccines against SARS-CoV-2 have demonstrated excellent safety profiles in clinical trials, real-world surveillance of post-vaccination side effects is an impetus. The study investigates the short-term side effects following the administration of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines in Saudi Arabia. A cross-sectional quantitative study was conducted among the general population with age ≥ 18 years, from five regions (Central, Northern, Eastern, Southern, and Western Regions) of Saudi Arabia for a period of 6 months (July to December 2021). A self-administered study instrument was used to record the side effects among the COVID-19 vaccine recipients. Of the total 398 participants (males: 59%), 56.3% received Pfizer and 43.7% were vaccinated with AstraZeneca. Only 22.6% of respondents received the second dose of the COVID-19 vaccines. The most commonly reported side effects were pain at the injection site (85.2%), fatigue (61.8%), bone or joint pain (54.0%), and fever (42.5%). The average side effects score was 3.4 ± 2.2. Females, young people, and Oxford-AstraZeneca recipients had a higher proportion of side effects. The Oxford-AstraZeneca vaccine recipients complained more about fever (p < 0.001), bone and joint pain (p < 0.001), fatigue (p < 0.001), loss of appetite (p = 0.001), headache (p = 0.008), and drowsiness (p = 0.003). The Pfizer-BioNTech vaccinees had more pain and swelling at the injection site (p = 0.001), and sexual disturbance (p = 0.019). The study participants also reported some rare symptoms (<10%) including heaviness, sleep disturbance, fainting, blurred vision, palpitations, osteomalacia, and inability to concentrate. This study revealed that both Pfizer-BioNTech and Oxford-AstraZeneca administration was associated with mild to moderate, transient, short-lived side effects. These symptoms corroborate the results of phase 3 clinical trials of these vaccines. The results could be used to inform people about the likelihood of side effects based on their demographics and the type of vaccine administered. The study reported some rare symptoms that require further validation through more pharmacovigilance or qualitative studies.
Keywords: COVID-19; Oxford-AstraZeneca; Pfizer-BioNTech; Saudi Arabia; pharmacovigilance; safety profile; side effects; surveillance; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Misbah S., Ahmad A., Butt M.H., Khan Y.H., Alotaibi N.H., Mallhi T.H. A systematic analysis of studies on corona virus disease 19 (COVID-19) from viral emergence to treatment. J. Coll. Physicians Surg. Pak. 2020;30:9–18. - PubMed
-
- Ferrante L., Duczmal L.H., Capanema E., Steinmetz W.A.C., Almeida A.C.L., Leão J., Vassão R.C., Fearnside P.M., Tupinambás U. Dynamics of COVID-19 in Amazonia: A history of government denialism and the risk of a third wave. Prev. Med. Rep. 2022;26:101752. doi: 10.1016/j.pmedr.2022.101752. - DOI - PMC - PubMed
-
- Christensen P.A., Olsen R.J., Long S.W., Subedi S., Davis J.J., Hodjat P., Walley D.R., Kinskey J.C., Saavedra M.O., Pruitt L. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am. J. Pathol. 2022;192:320–331. doi: 10.1016/j.ajpath.2021.10.019. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous