Immunological Study of Combined Administration of SARS-CoV-2 DNA Vaccine and Inactivated Vaccine
- PMID: 35746536
- PMCID: PMC9228235
- DOI: 10.3390/vaccines10060929
Immunological Study of Combined Administration of SARS-CoV-2 DNA Vaccine and Inactivated Vaccine
Abstract
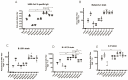
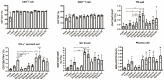
Objective: We constructed two DNA vaccines containing the receptor-binding domain (RBD) genes of multiple SARS-CoV-2 variants and used them in combination with inactivated vaccines in a variety of different protocols to explore potential novel immunization strategies against SARS-CoV-2 variants. Methods: Two DNA vaccine candidates with different signal peptides (namely, secreted and membrane signal peptides) and RBD protein genes of different SARS-CoV-2 strains (Wuhan-Hu-1, B.1.351, B.1.617.2, C.37) were used. Four different combinations of DNA and inactivated vaccines were tested, namely, Group A: three doses of DNA vaccine; B: three doses of DNA vaccine and one dose of inactivated vaccine; C: two doses of inactivated vaccine and one dose of DNA vaccine; and D: coadministration of DNA and inactivated vaccines in two doses. Subgroups were grouped according to the signal peptide used (subgroup 1 contained secreted signal peptides, and subgroup 2 contained membrane signal peptides). The in vitro expression of the DNA vaccines, the humoral and cellular immunity responses of the immunized mice, the immune cell population changes in local lymph nodes, and proinflammatory cytokine levels in serum samples were evaluated. Results: The antibody responses and cellular immunity in Group A were weak for all SARS-CoV-2 strains; for Group B, there was a great enhancement of neutralizing antibody (Nab) titers against the B.1.617.2 variant strain. Group C showed a significant increase in antibody responses (NAb titers against the Wuhan-Hu-1 strain were 768 and 1154 for Group C1 and Group C2, respectively, versus 576) and cellular immune responses, especially for variant B.1.617.2 (3240 (p < 0.001) and 2430 (p < 0.05) for Group C1 and Group C2, versus 450); Group D showed an improvement in immunogenicity. Group C induced higher levels of multiple cytokines. Conclusion: The DNA vaccine candidates we constructed, administered as boosters, could enhance the humoral and cellular immune responses of inactivated vaccines against COVID-19, especially for B.1.617.2.
Keywords: COVID-19; DNA vaccine; RBD; SARS-CoV-2; inactivated vaccine; variants.
Conflict of interest statement
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- World Health Organization Virtual Press Conference on COVID-19. 2020. [(accessed on 21 April 2022)]. Available online: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-aud....
-
- World Health Organization Home/Diseases/Coronavirus Disease (COVID-19) [(accessed on 21 April 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

