Design, Characterization, and Immune Augmentation of Docosahexaenoic Acid Nanovesicles as a Potential Delivery System for Recombinant HBsAg Protein
- PMID: 35746563
- PMCID: PMC9231307
- DOI: 10.3390/vaccines10060954
Design, Characterization, and Immune Augmentation of Docosahexaenoic Acid Nanovesicles as a Potential Delivery System for Recombinant HBsAg Protein
Abstract
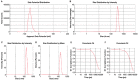
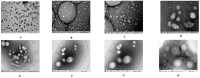
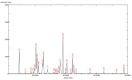
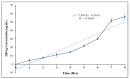
Recombinant HBsAg-loaded docosahexaenoic acid nanovesicles were successfully developed, lyophilized (LRPDNV) and characterized for their physico-chemical properties. The zetapotential (ZP) of LRPDNV was -60.4 ± 10.4 mV, and its polydispersity (PDI) was 0.201, with a % PDI of 74.8. The particle sizes of LRPDNV were 361.4 ± 48.24 z. d.nm and 298.8 ± 13.4 r.nm. The % mass (r.nm) of LRPDNV in a colloidal injectable system was 50, its mobility value was -3.417 µm cm/Vs, while the conductivity of the particles was 0.728 (mS/cm). Transmission electron microscopic (TEM) analysis showed smooth morphological characteristics of discrete spherical LRPDNV. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) of LRPDNV revealed that LRPDNV is thermostable. The X-ray diffraction (XRD) studies showed a discrete crystalline structure of LRPDNV at 2θ. Nuclear magnet resonance (NMR) studies (1H-NMR and 13C-NMR spectrum showed the discrete structure of LRPDNV. The immunogenicity study was performed by antibody induction technique. The anti-HBs IgG levels were elevated in Wistar rats; the antibody induction was observed more in the product (LRPDNV) treatment group when compared to the standard vaccine group. The level of antibodies on the 14th and 30th day was 6.3 ± 0.78 U/mL and 9.24 ± 1.76 U/mL in the treatment and standard vaccine groups, respectively. Furthermore, the antibody level on the 30th day in the treatment group was 26.66 ± 0.77 U/mL, and in the standard vaccine group, the antibody level was 23.94 ± 1.62 U/mL. The LRPDNV vaccine delivery method released HBsAg sustainably from the 14th to the 30th day. The results of this study indicate the successful formulation of DHA nanovesicles which have great potential as an adjuvant system for the delivery of recombinant HBsAg protein.
Keywords: HBsAg; docosahexaenoic acid; nanovesicles; recombinant proteins; vaccine adjuvants.
Conflict of interest statement
The authors declare that no conflict of interest is associated with this study, either financially or otherwise.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

