Immunogenicity Evaluating of the Multivalent COVID-19 Inactivated Vaccine against the SARS-CoV-2 Variants
- PMID: 35746564
- PMCID: PMC9228943
- DOI: 10.3390/vaccines10060956
Immunogenicity Evaluating of the Multivalent COVID-19 Inactivated Vaccine against the SARS-CoV-2 Variants
Abstract
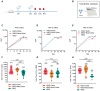
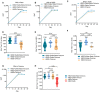
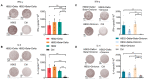
It has been reported that the novel coronavirus (COVID-19) has caused more than 286 million cases and 5.4 million deaths to date. Several strategies have been implemented globally, such as social distancing and the development of the vaccines. Several severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants have appeared, such as Alpha, Beta, Gamma, Delta, and Omicron. With the rapid spread of the novel coronavirus and the rapidly changing mutants, the development of a broad-spectrum multivalent vaccine is considered to be the most effective way to defend against the constantly mutating virus. Here, we evaluated the immunogenicity of the multivalent COVID-19 inactivated vaccine. Mice were immunized by multivalent COVID-19 inactivated vaccine, and the neutralizing antibodies in serum were analyzed. The results show that HB02 + Delta + Omicron trivalent vaccine could provide broad spectrum protection against HB02, Beta, Delta, and Omicron virus. Additionally, the different multivalent COVID-19 inactivated vaccines could enhance cellular immunity. Together, our findings suggest that the multivalent COVID-19 inactivated vaccine can provide broad spectrum protection against HB02 and other virus variants in humoral and cellular immunity, providing new ideas for the development of a broad-spectrum COVID-19 vaccine.
Keywords: Beta; Delta; HB02; Omicron; SARS-CoV-2; immunogenicity; multivalent inactivated vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Alum/CpG Adjuvanted Inactivated COVID-19 Vaccine with Protective Efficacy against SARS-CoV-2 and Variants.Vaccines (Basel). 2022 Jul 29;10(8):1208. doi: 10.3390/vaccines10081208. Vaccines (Basel). 2022. PMID: 36016098 Free PMC article.
-
Multivalent mRNA Vaccine Elicits Broad Protection against SARS-CoV-2 Variants of Concern.Vaccines (Basel). 2024 Jun 26;12(7):714. doi: 10.3390/vaccines12070714. Vaccines (Basel). 2024. PMID: 39066352 Free PMC article.
-
Nasal delivery of broadly neutralizing antibodies protects mice from lethal challenge with SARS-CoV-2 delta and omicron variants.Virol Sin. 2022 Apr;37(2):238-247. doi: 10.1016/j.virs.2022.02.005. Epub 2022 Feb 18. Virol Sin. 2022. PMID: 35527227 Free PMC article.
-
COVID-19 vaccines: their effectiveness against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its emerging variants.Bull Natl Res Cent. 2022;46(1):96. doi: 10.1186/s42269-022-00787-z. Epub 2022 Apr 8. Bull Natl Res Cent. 2022. PMID: 35431535 Free PMC article. Review.
-
Status Report on COVID-19 Vaccines Development.Curr Infect Dis Rep. 2021;23(6):9. doi: 10.1007/s11908-021-00752-3. Epub 2021 Apr 14. Curr Infect Dis Rep. 2021. PMID: 33867863 Free PMC article. Review.
Cited by
-
The importance of booster vaccination in the context of Omicron wave.Front Immunol. 2022 Sep 8;13:977972. doi: 10.3389/fimmu.2022.977972. eCollection 2022. Front Immunol. 2022. PMID: 36159796 Free PMC article. Review.
-
Development of variant-proof severe acute respiratory syndrome coronavirus 2, pan-sarbecovirus, and pan-β-coronavirus vaccines.J Med Virol. 2023 Jan;95(1):e28172. doi: 10.1002/jmv.28172. Epub 2022 Oct 6. J Med Virol. 2023. PMID: 36161303 Free PMC article. Review.
-
Development of highly adaptable RT-PCR methods for identifying Delta and BA.1 variants in inactivated COVID-19 vaccines.Mol Biol Rep. 2024 Aug 7;51(1):892. doi: 10.1007/s11033-024-09799-6. Mol Biol Rep. 2024. PMID: 39110319
-
Systematic Evaluation of the Distribution of Immune Cells following Subcutaneous Administration of Haemophilus Influenzae Type B Vaccine to Mice.Diseases. 2023 Oct 13;11(4):139. doi: 10.3390/diseases11040139. Diseases. 2023. PMID: 37873783 Free PMC article.
-
Innovation-driven trend shaping COVID-19 vaccine development in China.Front Med. 2023 Dec;17(6):1096-1116. doi: 10.1007/s11684-023-1034-6. Epub 2023 Dec 16. Front Med. 2023. PMID: 38102402 Review.
References
-
- Sheha Ta A.A., Parvin R., Nagy A., Wang Y., Rahmatullah A.K. An overview of ongoing challenges in SARS-CoV-2 global control. Ger. J. Microbiol. 2021;1:1–18. doi: 10.51585/gjm.2021.2.0006. - DOI
-
- Garcia-Beltran W.F., Lam E.C., St Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., Feldman J., Pavlovic M.N., Gregory D.J., Poznansky M.C., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383.e9. doi: 10.1016/j.cell.2021.03.013. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

