Adenovirus DNA Polymerase Loses Fidelity on a Stretch of Eleven Homocytidines during Pre-GMP Vaccine Preparation
- PMID: 35746566
- PMCID: PMC9227658
- DOI: 10.3390/vaccines10060960
Adenovirus DNA Polymerase Loses Fidelity on a Stretch of Eleven Homocytidines during Pre-GMP Vaccine Preparation
Abstract
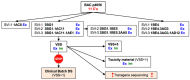
In this study, we invented and construct novel candidate HIV-1 vaccines. Through genetic and protein engineering, we unknowingly constructed an HIV-1-derived transgene with a homopolymeric run of 11 cytidines, which was inserted into an adenovirus vaccine vector. Here, we describe the virus rescue, three rounds of clonal purification and preparation of good manufacturing practise (GMP) starting material assessed for genetic stability in five additional virus passages. Throughout these steps, quality control assays indicated the presence of the transgene in the virus genome, expression of the correct transgene product and immunogenicity in mice. However, DNA sequencing of the transgene revealed additional cytidines inserted into the original 11-cytidine region, and the GMP manufacture had to be aborted. Subsequent analyses indicated that as little as 1/25th of the virus dose used for confirmation of protein expression (106 cells at a multiplicity of infection of 10) and murine immunogenicity (108 infectious units per animal) met the quality acceptance criteria. Similar frameshifts in the expressed proteins were reproduced in a one-reaction in vitro transcription/translation employing phage T7 polymerase and E. coli ribosomes. Thus, the most likely mechanism for addition of extra cytidines into the ChAdOx1.tHIVconsv6 genome is that the adenovirus DNA polymerase lost its fidelity on a stretch of 11 cytidines, which informs future adenovirus vaccine designs.
Keywords: HIVconsvX; T7 polymerase; adenovirus DNA polymerase; polymerase fidelity; protein engineering; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Plotkin S.A., Plotkin S. A Short History of Vaccination. 5th ed. Elsevier-Saunders; Philadelphia, PA, USA: 2008.
-
- Chong S.S.F., Kim M., Limoli M., Obscherning E., Wu P., Feisee L., Nakashima N., Lim J.C.W. Measuring progress of regulatory convergence and cooperation among Asia–Pacific Economic Cooperation (APEC) member economies in the context of the COVID-19 pandemic. Ther. Innov. Regul. Sci. 2021;55:786–798. doi: 10.1007/s43441-021-00285-w. - DOI - PMC - PubMed
Grants and funding
- HHSN27220110002II/HHSN27200037, Subcontract No.: OX-14007-004-0037-212/NH/NIH HHS/United States
- MR/N023668/1/MRC_/Medical Research Council/United Kingdom
- SRIA2015-1066/European & Developing Countries Clinical Trials Partnership
- 681137-EAVI2020/European Commission
- SOW5/International AIDS Vaccine Initiative
LinkOut - more resources
Full Text Sources
Miscellaneous

