Positive Attribute Framing Increases COVID-19 Booster Vaccine Intention for Unfamiliar Vaccines
- PMID: 35746570
- PMCID: PMC9229566
- DOI: 10.3390/vaccines10060962
Positive Attribute Framing Increases COVID-19 Booster Vaccine Intention for Unfamiliar Vaccines
Abstract
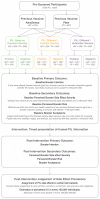
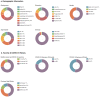
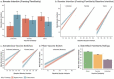
Positive framing has been proposed as an intervention to increase COVID-19 vaccination intentions. However, available research has examined fictitious or unfamiliar treatments. This pre-registered study (aspredicted#78369) compared the effect of standard negatively framed EU patient information leaflets (PILs), with new positively framed PILs, on booster intentions (measured pre- and post-intervention) for AstraZeneca, Pfizer, and Moderna COVID-19 vaccines. A representative sample of 1222 UK-based adults was randomised to one of six groups in a factorial design with framing (Positive vs. Negative) and vaccine familiarity (same (as previous), familiar, unfamiliar) as factors. The benefit of positive framing was hypothesised to be strongest for the least familiar vaccine (Moderna). Framing was moderated by familiarity, where only the unfamiliar vaccine showed a benefit of positive relative to negative Framing. Framing and familiarity also interacted with baseline Intention with the effect of framing on the unfamiliar vaccine especially pronounced at low baseline Intent. Conversely, standard negative framing appeared to increase intentions for familiar vaccines at low baseline intent. Findings provide important evidence that positive framing could improve vaccine uptake globally when switches or new developments require individuals to receive less familiar vaccines. Positive framing of familiar vaccines, however, should be treated with caution until better understood.
Keywords: COVID-19; attribute framing; positive framing; vaccination; vaccine hesitancy; vaccine intention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., Gallagher E., Chand M., Brown K., Ladhani S.N., et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. medRxiv. 2021 doi: 10.1101/2021.09.15.21263583v2. - DOI
-
- Cele S., Jackson L., Khan K., Khoury D.S., Moyo-Gwete T., Tegally H., Scheepers C., Amoako D., Karim F., Bernstein M., et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv. 2021 doi: 10.1101/2021.12.08.21267417. - DOI
-
- Basile K., Rockett R.J., McPhie K., Fennell M., Johnson-Mackinnon J., Agius J.E., Fong W., Rahman H., Ko D., Donavan L., et al. Improved neutralization of the SARS-CoV-2 Omicron variant after Pfizer-BioNTech BNT162b2 COVID-19 vaccine boosting. bioRxiv. 2021 doi: 10.1101/2021.12.12.472252. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

