Early IgE Production Is Linked with Extrafollicular B- and T-Cell Activation in Low-Dose Allergy Model
- PMID: 35746576
- PMCID: PMC9231339
- DOI: 10.3390/vaccines10060969
Early IgE Production Is Linked with Extrafollicular B- and T-Cell Activation in Low-Dose Allergy Model
Abstract
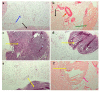
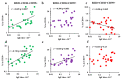
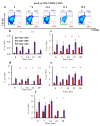
Despite its paramount importance, the predominant association of early IgE production with harmless antigens, via germinal-center B- and T-cell subpopulations or extrafollicular activation, remains unresolved. The aim of this work was to clarify whether the reinforced IgE production following the subcutaneous immunization of BALB/c mice with low antigen doses in withers adipose tissue might be linked with intensified extrafollicular or germinal-center responses. The mice were immunized three times a week for 4 weeks in the withers region, which is enriched in subcutaneous fat and tissue-associated B cells, with high and low OVA doses and via the intraperitoneal route for comparison. During long-term immunization with both low and high antigen doses in the withers region, but not via the intraperitoneal route, we observed a significant accumulation of B220-CD1d-CD5-CD19+ B-2 extrafollicular plasmablasts in the subcutaneous fat and regional lymph nodes but not in the intraperitoneal fat. Only low antigen doses induced a significant accumulation of CXCR4+ CXCR5- CD4+ extrafollicular T helpers in the withers adipose tissue but not in the regional lymph nodes or abdominal fat. Only in subcutaneous fat was there a combination of extrafollicular helper accumulation. In conclusion, extrafollicular B- and T-cell activation are necessary for early IgE class switching.
Keywords: IgE; extrafollicular T helpers; extrafollicular response; plasmablasts; subcutaneous fat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sweerus K., Lachowicz-Scroggins M., Gordon E., LaFemina M., Huang X., Parikh M., Kanegai C., Fahy J.V., Frank J.A. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J. Allergy Clin. Immunol. 2017;139:72–81. doi: 10.1016/j.jaci.2016.02.035. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

