Impact of DNA Prime/Protein Boost Vaccination against Campylobacter jejuni on Immune Responses and Gut Microbiota in Chickens
- PMID: 35746589
- PMCID: PMC9231206
- DOI: 10.3390/vaccines10060981
Impact of DNA Prime/Protein Boost Vaccination against Campylobacter jejuni on Immune Responses and Gut Microbiota in Chickens
Abstract
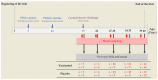
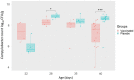
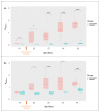
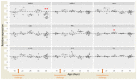
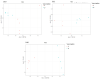
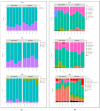
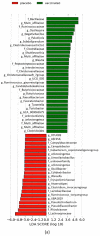
Campylobacteriosis is reported to be the leading zoonosis in Europe, and poultry is the main reservoir of Campylobacter. Despite all the efforts made, there is still no efficient vaccine to fight this bacterium directly in poultry. Recent studies have reported interactions between the chicken immune system and gut microbiota in response to Campylobacter colonisation. The present study was designed to analyse in more depth the immune responses and caecal microbiota following vaccination with a DNA prime/protein boost flagellin-based vaccine that induces some protection in specific-pathogen-free White Leghorn chickens, as shown previously. These data may help to improve future vaccination protocols against Campylobacter in poultry. Here a vaccinated and a placebo group were challenged by C. jejuni at the age of 19 days. A partial reduction in Campylobacter loads was observed in the vaccinated group. This was accompanied by the production of specific systemic and mucosal antibodies. Transient relatively higher levels of Interleukin-10 and antimicrobial peptide avian β-defensin 10 gene expressions were observed in the vaccinated and placebo groups respectively. The analysis of caecal microbiota revealed the vaccination's impact on its structure and composition. Specifically, levels of operational taxonomic units classified as Ruminococcaceae and Bacillaceae increased on day 40.
Keywords: Campylobacter jejuni caecal colonisation; caecal microbiota composition; flagellin antigen; innate immunity; poultry; systemic and mucosal immune response.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Hue O., Allain V., Laisney M.-J., Le Bouquin S., Lalande F., Petetin I., Rouxel S., Quesne S., Gloaguen P.-Y., Picherot M., et al. Campylobacter Contamination of Broiler Caeca and Carcasses at the Slaughterhouse and Correlation with Salmonella Contamination. Food Microbiol. 2011;28:862–868. doi: 10.1016/j.fm.2010.11.003. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

