Protective Immune Responses Induced by an mRNA-LNP Vaccine Encoding prM-E Proteins against Japanese Encephalitis Virus Infection
- PMID: 35746593
- PMCID: PMC9227124
- DOI: 10.3390/v14061121
Protective Immune Responses Induced by an mRNA-LNP Vaccine Encoding prM-E Proteins against Japanese Encephalitis Virus Infection
Abstract
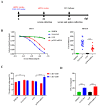
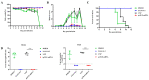
Japanese encephalitis virus (JEV) is an important zoonotic pathogen, which causes central nervous system symptoms in humans and reproductive disorders in swine. It has led to severe impacts on human health and the swine industry; however, there is no medicine available for treating yet. Therefore, vaccination is the best preventive measure for this disease. In the study, a modified mRNA vaccine expressing the prM and E proteins of the JEV P3 strain was manufactured, and a mouse model was used to assess its efficacy. The mRNA encoding prM and E proteins showed a high level of protein expression in vitro and were encapsulated into a lipid nanoparticle (LNP). Effective neutralizing antibodies and CD8+ T-lymphocytes-mediated immune responses were observed in vaccinated mice. Furthermore, the modified mRNA can protect mice from a lethal challenge with JEV and reduce neuroinflammation caused by JEV. This study provides a new option for the JE vaccine and lays a foundation for the subsequent development of a more efficient and safer JEV mRNA vaccine.
Keywords: Japanese encephalitis virus; immunogenicity; mRNA vaccine; prM-E protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Electroporation enhances protective immune response of a DNA vaccine against Japanese encephalitis in mice and pigs.Vaccine. 2016 Nov 11;34(47):5751-5757. doi: 10.1016/j.vaccine.2016.10.001. Epub 2016 Oct 13. Vaccine. 2016. PMID: 27743649
-
Construction and immune efficacy of recombinant pseudorabies virus expressing PrM-E proteins of Japanese encephalitis virus genotype І.Virol J. 2015 Dec 10;12:214. doi: 10.1186/s12985-015-0449-3. Virol J. 2015. PMID: 26651827 Free PMC article.
-
Immunization with pseudotype baculovirus expressing envelope protein of Japanese encephalitis virus elicits protective immunity in mice.J Gene Med. 2009 Feb;11(2):150-9. doi: 10.1002/jgm.1282. J Gene Med. 2009. Retraction in: J Gene Med. 2009 May;11(5):454. doi: 10.1002/jgm.1330. PMID: 19040206 Retracted.
-
IMOJEV(®): a Yellow fever virus-based novel Japanese encephalitis vaccine.Expert Rev Vaccines. 2010 Dec;9(12):1371-84. doi: 10.1586/erv.10.139. Expert Rev Vaccines. 2010. PMID: 21105774 Review.
-
Japanese encephalitis virus: Associated immune response and recent progress in vaccine development.Microb Pathog. 2019 Nov;136:103678. doi: 10.1016/j.micpath.2019.103678. Epub 2019 Aug 19. Microb Pathog. 2019. PMID: 31437579 Review.
Cited by
-
Research progress of mosquito-borne virus mRNA vaccines.Mol Ther Methods Clin Dev. 2024 Dec 12;33(1):101398. doi: 10.1016/j.omtm.2024.101398. eCollection 2025 Mar 13. Mol Ther Methods Clin Dev. 2024. PMID: 39834558 Free PMC article. Review.
-
Japanese Encephalitis: Risk of Emergence in the United States and the Resulting Impact.Viruses. 2023 Dec 28;16(1):54. doi: 10.3390/v16010054. Viruses. 2023. PMID: 38257754 Free PMC article.
-
Immortalized porcine mesangial cell line competent for the cultivation of Japanese encephalitis virus.J Vet Sci. 2025 Mar;26(2):e20. doi: 10.4142/jvs.24222. J Vet Sci. 2025. PMID: 40183907 Free PMC article.
-
Development of nucleic acid-based vaccines against dengue and other mosquito-borne flaviviruses: the past, present, and future.Front Immunol. 2025 Jan 7;15:1475886. doi: 10.3389/fimmu.2024.1475886. eCollection 2024. Front Immunol. 2025. PMID: 39840044 Free PMC article. Review.
-
Advancements in nanoparticle-based vaccine development against Japanese encephalitis virus: a systematic review.Front Immunol. 2024 Dec 20;15:1505612. doi: 10.3389/fimmu.2024.1505612. eCollection 2024. Front Immunol. 2024. PMID: 39759527 Free PMC article.
References
-
- Wu D., Chen X., Liu W., Fu S., Li F., Liang G., Yang G., Zheng H., Li J., Yin Z., et al. Emergence of Japanese encephalitis among adults 40 years of age or older in northern China: Epidemiological and clinical characteristics. Transbound. Emerg. Dis. 2021;68:3415–3423. doi: 10.1111/tbed.13945. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

