Evidence of RedOX Imbalance during Zika Virus Infection Promoting the Formation of Disulfide-Bond-Dependent Oligomers of the Envelope Protein
- PMID: 35746600
- PMCID: PMC9227265
- DOI: 10.3390/v14061131
Evidence of RedOX Imbalance during Zika Virus Infection Promoting the Formation of Disulfide-Bond-Dependent Oligomers of the Envelope Protein
Abstract
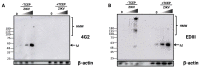
Flaviviruses replicate in membrane factories associated with the endoplasmic reticulum (ER). Significant levels of flavivirus viral protein accumulation contribute to ER stress. As a consequence, the host cell exhibits an Unfolded Protein Response (UPR), subsequently stimulating appropriate cellular responses such as adaptation, autophagy or apoptosis. The correct redox conditions of this compartment are essential to forming native disulfide bonds in proteins. Zika virus (ZIKV) has the ability to induce persistent ER stress leading to the activation of UPR pathways. In this study, we wondered whether ZIKV affects the redox balance and consequently the oxidative protein folding in the ER. We found that ZIKV replication influences the redox state, leading to the aggregation of the viral envelope protein as amyloid-like structures in the infected cells.
Keywords: ER stress; Zika virus; amyloid aggregates; disulfide bond; oligomer; unfolded protein response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Zika virus subversion of chaperone GRP78/BiP expression in A549 cells during UPR activation.Biochimie. 2020 Aug;175:99-105. doi: 10.1016/j.biochi.2020.05.011. Epub 2020 May 25. Biochimie. 2020. PMID: 32464166
-
Zika Virus Induces an Atypical Tripartite Unfolded Protein Response with Sustained Sensor and Transient Effector Activation and a Blunted BiP Response.mSphere. 2021 Jun 30;6(3):e0036121. doi: 10.1128/mSphere.00361-21. Epub 2021 Jun 9. mSphere. 2021. PMID: 34106769 Free PMC article.
-
BPIFB3 Regulates Endoplasmic Reticulum Morphology To Facilitate Flavivirus Replication.J Virol. 2020 Apr 16;94(9):e00029-20. doi: 10.1128/JVI.00029-20. Print 2020 Apr 16. J Virol. 2020. PMID: 32102874 Free PMC article.
-
Endoplasmic reticulum: a focal point of Zika virus infection.J Biomed Sci. 2020 Jan 20;27(1):27. doi: 10.1186/s12929-020-0618-6. J Biomed Sci. 2020. PMID: 31959174 Free PMC article. Review.
-
Role of autophagy in Zika virus infection and pathogenesis.Virus Res. 2018 Aug 2;254:34-40. doi: 10.1016/j.virusres.2017.09.006. Epub 2017 Sep 9. Virus Res. 2018. PMID: 28899653 Free PMC article. Review.
Cited by
-
Zika virus remodelled ER membranes contain proviral factors involved in redox and methylation pathways.Nat Commun. 2023 Dec 5;14(1):8045. doi: 10.1038/s41467-023-43665-6. Nat Commun. 2023. PMID: 38052817 Free PMC article.
-
Widespread amyloid aggregates formation by Zika virus proteins and peptides.Protein Sci. 2023 Dec;32(12):e4833. doi: 10.1002/pro.4833. Protein Sci. 2023. PMID: 37937856 Free PMC article.
References
-
- Miranda J., Martín-Tapia D., Valdespino-Vázquez Y., Alarcón L., Espejel-Nuñez A., Guzmán-Huerta M., Muñoz-Medina J.E., Shibayama M., Chávez-Munguía B., Estrada-Gutiérrez G., et al. Syncytiotrophoblast of Placentae from Women with Zika Virus Infection Has Altered Tight Junction Protein Expression and Increased Paracellular Permeability. Cells. 2019;8:1174. doi: 10.3390/cells8101174. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

