Organic Electrochemical Transistors as Versatile Tool for Real-Time and Automatized Viral Cytopathic Effect Evaluation
- PMID: 35746627
- PMCID: PMC9227436
- DOI: 10.3390/v14061155
Organic Electrochemical Transistors as Versatile Tool for Real-Time and Automatized Viral Cytopathic Effect Evaluation
Abstract
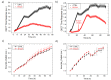
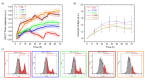
In-vitro viral studies are still fundamental for biomedical research since studying the virus kinetics on cells is crucial for the determination of the biological properties of viruses and for screening the inhibitors of infections. Moreover, testing potential viral contaminants is often mandatory for safety evaluation. Nowadays, viral cytopathic effects are mainly evaluated through end-point assays requiring dye-staining combined with optical evaluation. Recently, optical-based automatized equipment has been marketed, aimed at the real-time screening of cell-layer status and obtaining further insights, which are unavailable with end-point assays. However, these technologies present two huge limitations, namely, high costs and the possibility to study only cytopathic viruses, whose effects lead to plaque formation and layer disruption. Here, we employed poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (Pedot:Pss) organic electrochemical transistors (OECTs) for the real-time, electrical monitoring of the infection of cytolytic viruses, i.e., encephalomyocarditis virus (EMCV), and non-cytolytic viruses, i.e., bovine coronavirus (B-CoV), on cells. OECT data on EMCV were validated using a commercially-available optical-based technology, which, however, failed in the B-CoV titration analysis, as expected. The OECTs proved to be reliable, fast, and versatile devices for viral infection monitoring, which could be scaled up at low cost, reducing the operator workload and speeding up in-vitro assays in the biomedical research field.
Keywords: BCoV; ECMV; bovine coronavirus; cytolytic virus; encephalomyocarditis virus; non-cytolytic virus; organic electrochemical transistor; virus replication.
Conflict of interest statement
There is a patent pending on the reported technology, number 102021000023354. The inventors are Decataldo Francesco, Marta Tessarolo, Catia Giovannini, Vittorio Sambri, Alessandra Scagliarini, and Beatrice Fraboni. The remaining authors declare no competing interests.
Figures



Similar articles
-
Enhancement-Mode PEDOT:PSS Organic Electrochemical Transistors Using Molecular De-Doping.Adv Mater. 2020 May;32(19):e2000270. doi: 10.1002/adma.202000270. Epub 2020 Mar 23. Adv Mater. 2020. PMID: 32202010
-
Electrochemical Fabrication and Characterization of Organic Electrochemical Transistors Using poly(3,4-ethylenedioxythiophene) with Various Counterions.ACS Appl Mater Interfaces. 2022 Sep 21;14(37):42289-42297. doi: 10.1021/acsami.2c10149. Epub 2022 Sep 12. ACS Appl Mater Interfaces. 2022. PMID: 36095248
-
Biocompatible Ionic Liquids in High-Performing Organic Electrochemical Transistors for Ion Detection and Electrophysiological Monitoring.ACS Nano. 2022 Aug 23;16(8):12049-12060. doi: 10.1021/acsnano.2c02191. Epub 2022 Aug 8. ACS Nano. 2022. PMID: 35939084
-
Decoding Electrophysiological Signals with Organic Electrochemical Transistors.Macromol Biosci. 2021 Nov;21(11):e2100187. doi: 10.1002/mabi.202100187. Epub 2021 Aug 30. Macromol Biosci. 2021. PMID: 34463019 Review.
-
Functionalized Organic Thin Film Transistors for Biosensing.Acc Chem Res. 2019 Feb 19;52(2):277-287. doi: 10.1021/acs.accounts.8b00448. Epub 2019 Jan 8. Acc Chem Res. 2019. PMID: 30620566 Review.
Cited by
-
Organic Bioelectronics Development in Italy: A Review.Micromachines (Basel). 2023 Feb 16;14(2):460. doi: 10.3390/mi14020460. Micromachines (Basel). 2023. PMID: 36838160 Free PMC article. Review.
References
-
- Kim J.W., Murphy J., Chang A.L., Spencer D.A., Kane J.R., Kanojia D., Rashidi A., Young J.S., Lesniak M.S. All Aboard: Mesenchymal Stem/Stromal Cells as Cell Carriers for Virotherapy. Mesenchymal Stromal Cells Tumor Stromal Modul. 2017;19:475–499. doi: 10.1016/B978-0-12-803102-5.00019-7. - DOI
-
- Purtscher M., Rothbauer M., Kratz S.R.A., Bailey A., Lieberzeit P., Ertl P. A microfluidic impedance-based extended infectivity assay: Combining retroviral amplification and cytopathic effect monitoring on a single lab-on-a-chip platform. Lab Chip. 2021;21:1364–1372. doi: 10.1039/D0LC01056A. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

