Simian Varicella Virus Pathogenesis in Skin during Varicella and Zoster
- PMID: 35746639
- PMCID: PMC9227806
- DOI: 10.3390/v14061167
Simian Varicella Virus Pathogenesis in Skin during Varicella and Zoster
Abstract
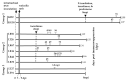
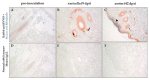
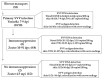
Primary simian varicella virus (SVV) infection and reactivation in nonhuman primates is a valuable animal model in the study of varicella zoster virus disease [varicella (chickenpox) and herpes zoster (shingles)]. To understand SVV pathogenesis in skin, we inoculated 10 rhesus macaques with SVV, resulting in varicella rash. After the establishment of latency, eight of the monkeys were immunosuppressed using tacrolimus with or without irradiation and prednisone and two monkeys were not immunosuppressed. Zoster rash developed in all immunosuppressed monkeys and in one non-immunosuppressed monkey. Five monkeys had recurrent zoster. During varicella and zoster, SVV DNA in skin scrapings ranged from 50 to 107 copies/100 ng of total DNA and 2-127 copies/100 ng of total DNA, respectively. Detection of SVV DNA in blood during varicella was more frequent and abundant compared to that of zoster. During varicella and zoster, SVV antigens colocalized with neurons expressing β-III tubulin in epidermis, hair follicles, and sweat glands, suggesting axonal transport of the virus. Together, we have demonstrated that both SVV DNA and antigens can be detected in skin lesions during varicella and zoster, providing the basis for further studies on SVV skin pathogenesis, including immune responses and mechanisms of peripheral spread.
Keywords: simian varicella virus; skin pathogenesis; varicella; zoster.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Kronenberg A., Bossart W., Wuthrich R.P., Cao C., Lautenschlager S., Wiegand N.D., Mullhaupt B., Noll G., Mueller N.J., Speck R.F. Retrospective analysis of varicella zoster virus (VZV) copy DNA numbers in plasma of immunocompetent patients with herpes zoster, of immunocompromised patients with disseminated VZV disease, and of asymptomatic solid organ transplant recipients. Transpl. Infect. Dis. 2005;7:116–121. doi: 10.1111/j.1399-3062.2005.00106.x. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

