MicroRNA Regulation of Human Herpesvirus Latency
- PMID: 35746686
- PMCID: PMC9231095
- DOI: 10.3390/v14061215
MicroRNA Regulation of Human Herpesvirus Latency
Abstract
Herpesviruses are ubiquitous human pathogens. After productive (lytic) infection, all human herpesviruses are able to establish life-long latent infection and reactivate from it. Latent infection entails suppression of viral replication, maintenance of the viral genome in infected cells, and the ability to reactivate. Most human herpesviruses encode microRNAs (miRNAs) that regulate these processes during latency. Meanwhile, cellular miRNAs are hijacked by herpesviruses to participate in these processes. The viral or cellular miRNAs either directly target viral transcripts or indirectly affect viral infection through host pathways. These findings shed light on the molecular determinants that control the lytic-latent switch and may lead to novel therapeutics targeting latent infection. We discuss the multiple mechanisms by which miRNAs regulate herpesvirus latency, focusing on the patterns in these mechanisms.
Keywords: EBV; HCMV; HSV; KSHV; herpesvirus; latency; microRNA; reactivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
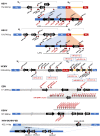

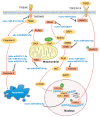

References
-
- Arbuckle J.H., Medveczky M.M., Luka J., Hadley S.H., Luegmayr A., Ablashi D., Lund T.C., Tolar J., De Meirleir K., Montoya J.G., et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 2010;107:5563–5568. doi: 10.1073/pnas.0913586107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

