The Isolation and Full-Length Transcriptome Sequencing of a Novel Nidovirus and Response of Its Infection in Japanese Flounder (Paralichthys olivaceus)
- PMID: 35746687
- PMCID: PMC9230003
- DOI: 10.3390/v14061216
The Isolation and Full-Length Transcriptome Sequencing of a Novel Nidovirus and Response of Its Infection in Japanese Flounder (Paralichthys olivaceus)
Abstract
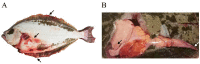
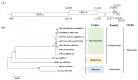
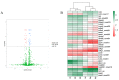
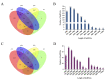
A novel nidovirus, CSBV Bces-Po19, was isolated from the marine fish, Japanese flounder (Paralichthys olivaceus). The viral genome was 26,597 nucleotides long and shared 98.62% nucleotide identity with CSBV WHQSR4345. PacBio Sequel and Illumina sequencing were used to perform full-length transcriptome sequencing on CSBV Bces-Po19-sensitive (S) and -resistant (R) Japanese flounder. The results of negative staining revealed bacilliform and spherical virions. There were in total 1444 different genes between CSBV Bces-Po19 S and R groups, with 935 being up-regulated and 513 being down-regulated. Metabolism-, immune-, and RNA-related pathways were significantly enriched. Furthermore, CSBV Bces-Po19 infection induced alternative splicing (AS) events in Japanese flounder; the S group had a higher numbers of AS events (12,352) than the R group (11,452). The number of long non-coding RNA (lncRNA) in the S group, on the other hand, was significantly lower than in the R group. In addition to providing valuable information that sheds more light on CSBV Bces-Po19 infection, these research findings provide further clues for CSBV Bces-Po19 prevention and treatment.
Keywords: alternative splicing; isoform sequencing; marine fish; metabolism; viral genome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Chen X.-Y., Zhou Y., Chen X., Zheng J., Zeng X.-D., Ji F. Isolation and genetic analysis of a nidovirus from crucian carp (Carassius auratus) Arch. Virol. 2019;164:1651–1654. - PubMed
-
- Walker P.J., Siddell S.G., Lefkowitz E.J., Mushegian A.R., Adriaenssens E.M., Alfenas-Zerbini P., Davison A.J., Dempsey D.M., Dutilh B.E., García M.L., et al. Changes to virus taxonomy and to the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2021) Arch. Virol. 2021;166:2633–2648. doi: 10.1007/s00705-021-05156-1. - DOI - PubMed
-
- Cano I., Stone D., Savage J., Wood G., Mulhearn B., Gray J., Stinton N., Ross S., Bonar M., Taylor N.G.H., et al. Isolation of a Chinook Salmon Bafinivirus (CSBV) in Imported Goldfish Carassius auratus L. in the United Kingdom and Evaluation of Its Virulence in Resident Fish Species. Viruses. 2020;12:578. doi: 10.3390/v12050578. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

