New p35 (H3L) Epitope Involved in Vaccinia Virus Neutralization and Its Deimmunization
- PMID: 35746695
- PMCID: PMC9227246
- DOI: 10.3390/v14061224
New p35 (H3L) Epitope Involved in Vaccinia Virus Neutralization and Its Deimmunization
Abstract
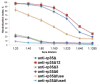
Vaccinia virus (VACV) is a promising oncolytic agent because it exhibits many characteristic features of an oncolytic virus. However, its effectiveness is limited by the strong antiviral immune response induced by this virus. One possible approach to overcome this limitation is to develop deimmunized recombinant VACV. It is known that VACV p35 is a major protein for B- and T-cell immune response. Despite the relevance of p35, its epitope structure remains insufficiently studied. To determine neutralizing epitopes, a panel of recombinant p35 variants was designed, expressed, and used for mice immunization. Plaque-reduction neutralization tests demonstrated that VACV was only neutralized by sera from mice that were immunized with variants containing both N- and C- terminal regions of p35. This result was confirmed by the depletion of anti-p35 mice sera with recombinant p35 variants. At least nine amino acid residues affecting the immunogenic profile of p35 were identified. Substitutions of seven residues led to disruption of B-cell epitopes, whereas substitutions of two residues resulted in the recognition of the mutant p35 solely by non-neutralizing antibodies.
Keywords: H3L; deimmunization; epitope; immunogenicity; neutralizing antibodies; oncolytic vaccinia virus; oncolytic virus; orthopoxvirus; p35; vaccinia virus.
Conflict of interest statement
All co-authors have seen and agree with the contents of the manuscript and the order of authors, and there is no financial interest to report. All co-authors declare that they have no conflict of interest.
Figures




Similar articles
-
Phage display antibodies against ectromelia virus that neutralize variola virus: Selection and implementation for p35 neutralizing epitope mapping.Antiviral Res. 2018 Apr;152:18-25. doi: 10.1016/j.antiviral.2018.02.006. Epub 2018 Feb 7. Antiviral Res. 2018. PMID: 29427674
-
Potent neutralization of vaccinia virus by divergent murine antibodies targeting a common site of vulnerability in L1 protein.J Virol. 2014 Oct;88(19):11339-55. doi: 10.1128/JVI.01491-14. Epub 2014 Jul 16. J Virol. 2014. PMID: 25031354 Free PMC article.
-
An epitope conserved in orthopoxvirus A13 envelope protein is the target of neutralizing and protective antibodies.Virology. 2011 Sep 15;418(1):67-73. doi: 10.1016/j.virol.2011.06.029. Epub 2011 Aug 2. Virology. 2011. PMID: 21810533 Free PMC article.
-
Design and Engineering of Deimmunized Vaccinia Viral Vectors.Biomedicines. 2020 Nov 11;8(11):491. doi: 10.3390/biomedicines8110491. Biomedicines. 2020. PMID: 33187060 Free PMC article. Review.
-
GP4-specific neutralizing antibodies might be a driving force in PRRSV evolution.Virus Res. 2010 Dec;154(1-2):104-13. doi: 10.1016/j.virusres.2010.08.026. Epub 2010 Sep 15. Virus Res. 2010. PMID: 20837070 Review.
Cited by
-
Immunization of mice with vaccinia virus Tiantan strain yields antibodies cross-reactive with protective antigens of monkeypox virus.Virol Sin. 2023 Feb;38(1):162-164. doi: 10.1016/j.virs.2022.10.004. Epub 2022 Oct 19. Virol Sin. 2023. PMID: 36272712 Free PMC article.
-
Identification of neutralizing nanobodies protecting against poxvirus infection.Cell Discov. 2025 Mar 25;11(1):31. doi: 10.1038/s41421-025-00771-7. Cell Discov. 2025. PMID: 40133273 Free PMC article.
-
Which Proteins? The Challenge of Identifying the Protective Antigens for Next-Generation Capripoxvirus Vaccines.Vaccines (Basel). 2025 Feb 22;13(3):219. doi: 10.3390/vaccines13030219. Vaccines (Basel). 2025. PMID: 40266091 Free PMC article. Review.
-
A robust comprehensive immunoinformatics approach for designing a potential multi-epitope based vaccine against a reiterated monkeypox virus.Biochem Biophys Rep. 2025 Jun 12;43:102075. doi: 10.1016/j.bbrep.2025.102075. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40583904 Free PMC article.
-
Monkeypox virus H3L protein as the target antigen for developing neutralizing antibody and serological assay.Appl Microbiol Biotechnol. 2025 Apr 2;109(1):80. doi: 10.1007/s00253-025-13466-6. Appl Microbiol Biotechnol. 2025. PMID: 40172630 Free PMC article.
References
-
- Moss B. Poxviridae. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. pp. 2129–2159.
-
- MacNeil A., Reynolds M.G., Carroll D.S., Karem K., Braden Z., Lash R., Moundeli A., Mombouli J.-V., Jumaan A.O., Schmid D.S., et al. Monkeypox or Varicella? Lessons from a Rash Outbreak Investigation in the Republic of the Congo. Am. J. Trop. Med. Hyg. 2009;80:503–507. doi: 10.4269/ajtmh.2009.80.503. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

