Azelnidipine Exhibits In Vitro and In Vivo Antiviral Effects against Flavivirus Infections by Targeting the Viral RdRp
- PMID: 35746699
- PMCID: PMC9230735
- DOI: 10.3390/v14061228
Azelnidipine Exhibits In Vitro and In Vivo Antiviral Effects against Flavivirus Infections by Targeting the Viral RdRp
Abstract
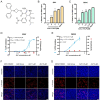
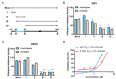
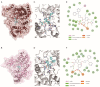
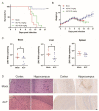
Flaviviruses, represented by Zika and dengue virus (ZIKV and DENV), are widely present around the world and cause various diseases with serious consequences. However, no antiviral drugs have been clinically approved for use against them. Azelnidipine (ALP) is a dihydropyridine calcium channel blocker and has been approved for use as an antihypertensive drug. In the present study, ALP was found to show potent anti-flavivirus activities in vitro and in vivo. ALP effectively prevented the cytopathic effect induced by ZIKV and DENV and inhibited the production of viral RNA and viral protein in a dose-dependent manner. Moreover, treatment with 0.3 mg/kg of ALP protected 88.89% of mice from lethal challenge. Furthermore, using the time-of-drug-addition assay, the enzymatic inhibition assay, the molecular docking, and the surface plasmon resonance assay, we revealed that ALP acted at the replication stage of the viral infection cycle by targeting the viral RNA-dependent RNA polymerase. These findings highlight the potential for the use of ALP as an antiviral agent to combat flavivirus infections.
Keywords: RdRp; Zika virus; antiviral; azelnidipine; flavivirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

