Interplay of HCPro and CP in the Regulation of Potato Virus A RNA Expression and Encapsidation
- PMID: 35746704
- PMCID: PMC9227828
- DOI: 10.3390/v14061233
Interplay of HCPro and CP in the Regulation of Potato Virus A RNA Expression and Encapsidation
Abstract
Potyviral coat protein (CP) and helper component-proteinase (HCPro) play key roles in both the regulation of viral gene expression and the formation of viral particles. We investigated the interplay between CP and HCPro during these viral processes. While the endogenous HCPro and a heterologous viral suppressor of gene silencing both complemented HCPro-less potato virus A (PVA) expression, CP stabilization connected to particle formation could be complemented only by the cognate PVA HCPro. We found that HCPro relieves CP-mediated inhibition of PVA RNA expression likely by enabling HCPro-mediated sequestration of CPs to particles. We addressed the question about the role of replication in formation of PVA particles and gained evidence for encapsidation of non-replicating PVA RNA. The extreme instability of these particles substantiates the need for replication in the formation of stable particles. During replication, viral protein genome linked (VPg) becomes covalently attached to PVA RNA and can attract HCPro, cylindrical inclusion protein and host proteins. Based on the results of the current study and our previous findings we propose a model in which a large ribonucleoprotein complex formed around VPg at one end of PVA particles is essential for their integrity.
Keywords: coat protein (CP); encapsidation; helper-component proteinase (HCPro); particle stability; potato virus A; potyvirus; viral gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
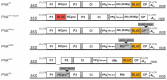



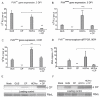
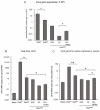
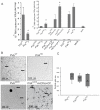


References
-
- González-Jara P., Atencio F.A., Martínez-García B., Barajas D., Tenllado F., Díaz-Ruíz J.R. A single amino acid mutation in the plum pox virus helper component-proteinase gene abolishes both synergistic and RNA silencing suppression activities. Phytopathology. 2005;95:894–901. doi: 10.1094/PHYTO-95-0894. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

