Intraneuronal β-Amyloid Accumulation: Aging HIV-1 Human and HIV-1 Transgenic Rat Brain
- PMID: 35746739
- PMCID: PMC9230035
- DOI: 10.3390/v14061268
Intraneuronal β-Amyloid Accumulation: Aging HIV-1 Human and HIV-1 Transgenic Rat Brain
Abstract
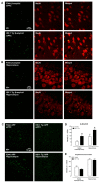
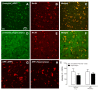
The prevalence of HIV-1 associated neurocognitive disorders (HAND) is significantly greater in older, relative to younger, HIV-1 seropositive individuals; the neural pathogenesis of HAND in older HIV-1 seropositive individuals, however, remains elusive. To address this knowledge gap, abnormal protein aggregates (i.e., β-amyloid) were investigated in the brains of aging (>12 months of age) HIV-1 transgenic (Tg) rats. In aging HIV-1 Tg rats, double immunohistochemistry staining revealed abnormal intraneuronal β-amyloid accumulation in the prefrontal cortex (PFC) and hippocampus, relative to F344/N control rats. Notably, in HIV-1 Tg animals, increased β-amyloid accumulation occurred in the absence of any genotypic changes in amyloid precursor protein (APP). Furthermore, no clear amyloid plaque deposition was observed in HIV-1 Tg animals. Critically, β-amyloid was co-localized with neurons in the cortex and hippocampus, supporting a potential mechanism underlying synaptic dysfunction in the HIV-1 Tg rat. Consistent with these neuropathological findings, HIV-1 Tg rats exhibited prominent alterations in the progression of temporal processing relative to control animals; temporal processing relies, at least in part, on the integrity of the PFC and hippocampus. In addition, in post-mortem HIV-1 seropositive individuals with HAND, intraneuronal β-amyloid accumulation was observed in the dorsolateral PFC and hippocampal dentate gyrus. Consistent with observations in the HIV-1 Tg rat, no amyloid plaques were found in these post-mortem HIV-1 seropositive individuals with HAND. Collectively, intraneuronal β-amyloid aggregation observed in the PFC and hippocampus of HIV-1 Tg rats supports a potential factor underlying HIV-1 associated synaptodendritic damage. Further, the HIV-1 Tg rat provides a biological system to model HAND in older HIV-1 seropositive individuals.
Keywords: HIV-1; RNAscope; neurodegenerative diseases; prepulse inhibition; β-amyloid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A novel transgenic rat model with a full Alzheimer's-like amyloid pathology displays pre-plaque intracellular amyloid-beta-associated cognitive impairment.J Alzheimers Dis. 2010;20(1):113-26. doi: 10.3233/JAD-2010-1349. J Alzheimers Dis. 2010. PMID: 20164597
-
Age-related amyloid beta deposition in transgenic mice overexpressing both Alzheimer mutant presenilin 1 and amyloid beta precursor protein Swedish mutant is not associated with global neuronal loss.Am J Pathol. 2000 Jul;157(1):331-9. doi: 10.1016/s0002-9440(10)64544-0. Am J Pathol. 2000. PMID: 10880403 Free PMC article.
-
A Gap in Time: Extending our Knowledge of Temporal Processing Deficits in the HIV-1 Transgenic Rat.J Neuroimmune Pharmacol. 2017 Mar;12(1):171-179. doi: 10.1007/s11481-016-9711-8. Epub 2016 Oct 3. J Neuroimmune Pharmacol. 2017. PMID: 27699630 Free PMC article.
-
Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer's disease.Pathol Int. 2017 Apr;67(4):185-193. doi: 10.1111/pin.12520. Epub 2017 Mar 5. Pathol Int. 2017. PMID: 28261941 Review.
-
APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review.
Cited by
-
Levetiracetam Prevents Neurophysiological Changes and Preserves Cognitive Function in the Human Immunodeficiency Virus (HIV)-1 Transactivator of Transcription Transgenic Mouse Model of HIV-Associated Neurocognitive Disorder.J Pharmacol Exp Ther. 2024 Sep 18;391(1):104-118. doi: 10.1124/jpet.124.002272. J Pharmacol Exp Ther. 2024. PMID: 39060163
-
HIV-Associated Insults Modulate ADAM10 and Its Regulator Sirtuin1 in an NMDA Receptor-Dependent Manner.Cells. 2022 Sep 22;11(19):2962. doi: 10.3390/cells11192962. Cells. 2022. PMID: 36230925 Free PMC article.
References
-
- HIV and Aging. [(accessed on 14 December 2021)]. Available online: https://www.unaids.org/en/resources/documents/2013/20131101_JC2563_hiv-a....
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS100624/NS/NINDS NIH HHS/United States
- U24 MH100925/MH/NIMH NIH HHS/United States
- U24 MH100931/MH/NIMH NIH HHS/United States
- MH106392/NH/NIH HHS/United States
- U24MH100930/NH/NIH HHS/United States
- DA013137/NH/NIH HHS/United States
- U24MH100931/NH/NIH HHS/United States
- R01 MH106392/MH/NIMH NIH HHS/United States
- K99 DA056288/DA/NIDA NIH HHS/United States
- U24MH100925/NH/NIH HHS/United States
- NS100624/NH/NIH HHS/United States
- R01 DA013137/DA/NIDA NIH HHS/United States
- U24 MH100930/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

