Antigenic Site Immunodominance Redirection Following Repeat Variant Exposure
- PMID: 35746763
- PMCID: PMC9229260
- DOI: 10.3390/v14061293
Antigenic Site Immunodominance Redirection Following Repeat Variant Exposure
Abstract
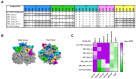
Human norovirus is a leading cause of acute gastroenteritis, driven by antigenic variants within the GII.4 genotype. Antibody responses to GII.4 vaccination in adults are shaped by immune memory. How children without extensive immune memory will respond to GII.4 vaccination has not been reported. Here, we characterized the GII.4 neutralizing antibody (nAb) landscape following natural infection using a surrogate assay and antigenic site chimera virus-like particles. We demonstrate that the nAb landscape changes with age and virus exposure. Among sites A, C, and G, nAbs from first infections are focused on sites A and C. As immunity develops with age/exposure, site A is supplemented with antibodies that bridge site A to sites C and G. Cross-site nAbs continue to develop into adulthood, accompanied by an increase in nAb to site G. Continued exposure to GII.4 2012 Sydney correlated with a shift to co-dominance of sites A and G. Furthermore, site G nAbs correlated with the broadening of nAb titer across antigenically divergent variants. These data describe fundamental steps in the development of immunity to GII.4 over a lifetime, and illustrate how the antigenicity of one pandemic variant could influence the pandemic potential of another variant through the redirection of immunodominant epitopes.
Keywords: antigenic seniority; blockade antibody; immune imprinting; immunodominance; neutralizing antibody; norovirus; variant persistence; variants of concern.
Conflict of interest statement
L.C.L. and R.S.B. hold patents on norovirus vaccine design and ongoing collaborations with Vaxart, Takeda Vaccines, and HilleVax that are unrelated and do not pose conflict of interest with this report. R.S.B. is a member of the advisory committee for Vaxart and Adagio Therapeutics. S.B.-D. received an investigator-initiated research award from Takeda Vaccines unrelated to this report. P.B.J., M.L.M., M.R.Z., S.R.M., J.B., D.A., R.W., D.K. and F.B. have no conflict of interest to report.
Figures


References
-
- Burke R.M., Mattison C., Marsh Z., Shioda K., Donald J., Salas S.B., Naleway A.L., Biggs C., Schmidt M.A., Hall A.J. Norovirus and Other Viral Causes of Medically Attended Acute Gastroenteritis Across the Age Spectrum: Results from the MAAGE Study in the United States. Clin. Infect. Dis. 2021;73:e913–e920. doi: 10.1093/cid/ciab033. - DOI - PMC - PubMed
-
- Wu C.Y., Chi H., Liu C.C., Huang Y.C., Huang Y.C., Lin H.C., Ho Y.H., Huang L.M., Huang C.Y., Shih S.M., et al. Clinical characteristics and risk factors for children with norovirus gastroenteritis in Taiwan. J. Microbiol. Immunol. Infect. 2020;54:909–917. doi: 10.1016/j.jmii.2020.07.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

