Influenza Virus Infections in Polarized Cells
- PMID: 35746778
- PMCID: PMC9231244
- DOI: 10.3390/v14061307
Influenza Virus Infections in Polarized Cells
Abstract
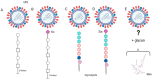
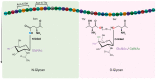
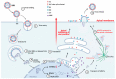
In humans and other mammals, the respiratory tract is represented by a complex network of polarized epithelial cells, forming an apical surface facing the external environment and a basal surface attached to the basement layer. These cells are characterized by differential expression of proteins and glycans, which serve as receptors during influenza virus infection. Attachment between these host receptors and the viral surface glycoprotein hemagglutinin (HA) initiates the influenza virus life cycle. However, the virus receptor binding specificities may not be static. Sialylated N-glycans are the most well-characterized receptors but are not essential for the entry of influenza viruses, and other molecules, such as O-glycans and non-sialylated glycans, may be involved in virus-cell attachment. Furthermore, correct cell polarity and directional trafficking of molecules are essential for the orderly development of the system and affect successful influenza infection; on the other hand, influenza infection can also change cell polarity. Here we review recent advances in our understanding of influenza virus infection in the respiratory tract of humans and other mammals, particularly the attachment between the virus and the surface of the polar cells and the polarity variation of these cells due to virus infection.
Keywords: N-glycan; O-glycan; influenza A virus; polarized cell; sialic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Walker P.J., Siddell S.G., Lefkowitz E.J., Mushegian A.R., Adriaenssens E.M., Dempsey D.M., Dutilh B.E., Harrach B., Harrison R.L., Hendrickson R.C., et al. Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses (2020) Arch. Virol. 2020;165:2737–2748. doi: 10.1007/s00705-020-04752-x. - DOI - PubMed
-
- Guo Y.J., Jin F.G., Wang P., Wang M., Zhu J.M. Isolation of influenza C virus from pigs and experimental infection of pigs with influenza C virus. Pt 1J. Gen. Virol. 1983;64:177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

