Immunometabolic Reprogramming in Response to HIV Infection Is Not Fully Normalized by Suppressive Antiretroviral Therapy
- PMID: 35746785
- PMCID: PMC9228482
- DOI: 10.3390/v14061313
Immunometabolic Reprogramming in Response to HIV Infection Is Not Fully Normalized by Suppressive Antiretroviral Therapy
Abstract
Background: HIV infection results in immunometabolic reprogramming. While we are beginning to understand how this metabolic reprogramming regulates the immune response to HIV infection, we do not currently understand the impact of ART on immunometabolism in people with HIV (PWH).
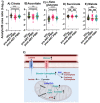
Methods: Serum obtained from HIV-infected (n = 278) and geographically matched HIV seronegative control subjects (n = 300) from Rakai Uganda were used in this study. Serum was obtained before and ~2 years following the initiation of ART from HIV-infected individuals. We conducted metabolomics profiling of the serum and focused our analysis on metabolic substrates and pathways assocaited with immunometabolism.
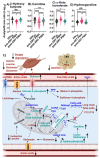
Results: HIV infection was associated with metabolic adaptations that implicated hyperactive glycolysis, enhanced formation of lactate, increased activity of the pentose phosphate pathway (PPP), decreased β-oxidation of long-chain fatty acids, increased utilization of medium-chain fatty acids, and enhanced amino acid catabolism. Following ART, serum levels of ketone bodies, carnitine, and amino acid metabolism were normalized, however glycolysis, PPP, lactate production, and β-oxidation of long-chain fatty acids remained abnormal.
Conclusion: Our findings suggest that HIV infection is associated with an increased immunometabolic demand that is satisfied through the utilization of alternative energetic substrates, including fatty acids and amino acids. ART alone was insufficient to completely restore this metabolic reprogramming to HIV infection, suggesting that a sustained impairment of immunometabolism may contribute to chronic immune activation and comorbid conditions in virally suppressed PWH.
Keywords: HIV infection; amino acid catabolism; antiretroviral therapy; comorbid conditions; fatty acid oxidation; glucose oxidation; immunometabolism.
Conflict of interest statement
The authors declare no conflict to interest.
Figures








References
-
- Valle-Casuso J.C., Angin M., Volant S., Passaes C., Monceaux V., Mikhailova A., Bourdic K., Avettand-Fenoel V., Boufassa F., Sitbon M., et al. Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4(+) T Cells and Offers an Opportunity to Tackle Infection. Cell Metab. 2019;29:611–626.e5. doi: 10.1016/j.cmet.2018.11.015. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

